Numab AG bags CHF70m in licencing deal
Swiss immunology and immuno-oncology specialist Numab Innovation AG has licenced its tri-specific antibody ND016 to Boston-based Intarcia Therapeutics Inc.
Under the agreement, Intarcia Therapeutics will pay up to CHF70m to gain exclusive, global rights for commercialisation of the preclinical tri-specific Fv antibody fragment candidate ND016. ND016 inhibits the pro-inflammatory cytokines IL-17A and TNF?, and binds to serum albumin. If ND016 makes it to the market Numab is eglible to receive double-digit tiered royalties.
ND016 is designed for the treatment of severe autoimmune disorders such as rheumatoid or psoriac arthritis. Under a previous 2015 option agreement, Intarcia and Numab agreed to combine Numab’s multifunction antibody fragments in three application fields (diabetes, obesity and autoimmune diseases) with its implantable drug delivery device Duros, which would theoretically allow a once- or twice-yearly therapy. Under the licence deal, Intarcia exercised this option.
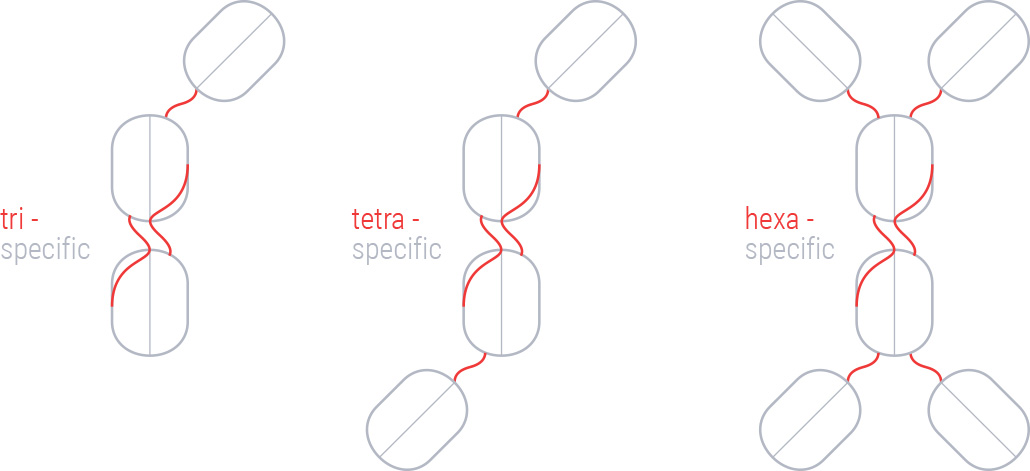
Usually, antibody Fv fragments are inherently instable. Numab has overcome this problem by replacing a piece of a human variable light kappa-chain with the equivalent lambda-chain stretch at the C-terminus of the light chain resulting in a stable human Fv acceptor scaffold to which up to 6 CDRs can be attached. By targeting multiple targets with one molecule, Numab hopes to overcome escape mechanisms of solid tumours and autoimmune diseases.


 Unsplash+
Unsplash+
