Novartis’ Ianalumab achieves Phase III endpoint in Sjögren’s syndrome
Novartis AG has reported a clinical milestone in the treatment of Sjögren’s syndrome, with its BAFF receptor blocker Ianalumab (VAY736) becoming the first investigational therapy to demonstrate a statistically significant reduction in disease activity in pivotal Phase III trials, as measured by the ESSDAI score.
In the company-sponsored NEPTUNUS‑1 and NEPTUNUS‑2 studies, Ianalumab—originally developed by MorphoSys AG—met its primary endpoint in adult patients with active Sjögren’s syndrome. The trials showed a statistically significant reduction in systemic disease activity versus placebo over 52 weeks, using 300 mg doses administered either monthly or quarterly. Novartis now plans to file for regulatory approval globally.
Ianalumab is a novel, fully human monoclonal antibody that exerts its effect by blocking the BAFF receptor and selectively depleting BAFF‑R–positive B cells. The trials used the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) to assess efficacy.
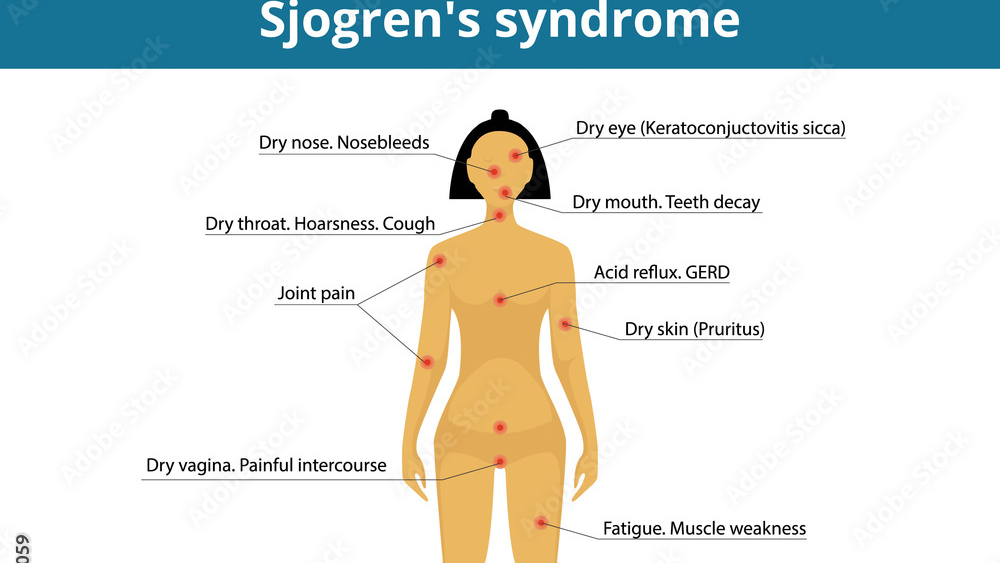
Sjögren’s syndrome is a chronic, systemic autoimmune disease with an estimated prevalence of 0.2%, primarily affecting exocrine glands but also other organs in around 30% of patients due to extraglandular manifestations. Analysts at AI Invest estimate the global commercial potential of Ianalumab at up to US$10bn by 2030.
While development in hidradenitis suppurativa was discontinued following negative Phase IIb results, Novartis continues to investigate Ianalumab across multiple autoimmune indications, including lupus nephritis, systemic lupus erythematosus (SLE), immune thrombocytopenia (ITP), rheumatoid arthritis, autoimmune hepatitis, multiple sclerosis, and pemphigus vulgaris.


 Immunic/Nela Dorner
Immunic/Nela Dorner adobe stock photos - Michael Derrer Fuchs
adobe stock photos - Michael Derrer Fuchs 