Novartis enters SMA race acquiring AveXis for US$8.7bn
Swiss drug major Novartis has secured a potential stake in the multibillion dollar market of treatments for the orphan genetic muscle disease spinal muscular atrophy (SMA). The company acquired AveXis Inc, whose SMA gene therapy AVXS-101 is in Phase III testing.
With the US$8.7bn take over, Novartis challenges Biogen’s US and EU approved antisense oligonucleotide Spinraza (nusinersen), which has been marketed by Biogen since 2016/2017 at an annual price of US$750,000 per patient. The splicing modifier originally developed by Ionis Pharmaceuticals turns SMN2 mRNA into a functional form of the defective SMN1 version. Swiss Roche is also heading for market approval with a splicing modifier of SMN2 code-named RG7916 in pivotal clinical trials. With LMI070, Novartis has also a modulator of SMN2 in Phase III testing to treat SMA.
However, the AveXis take-over is adding a gene therapy approach to the drug major’s portfolio that can be also used to correct other inherited orphan diseases without treatment option: besides AVXS-101, which delivers a functional copy of the deficient SMN gene, AveXis’ adeno-associated viral vector portfolio includes preclinical assets to treat patients with Rett syndrome and SOD2-deficient amyotrophic lateral sclerosis (ALS).
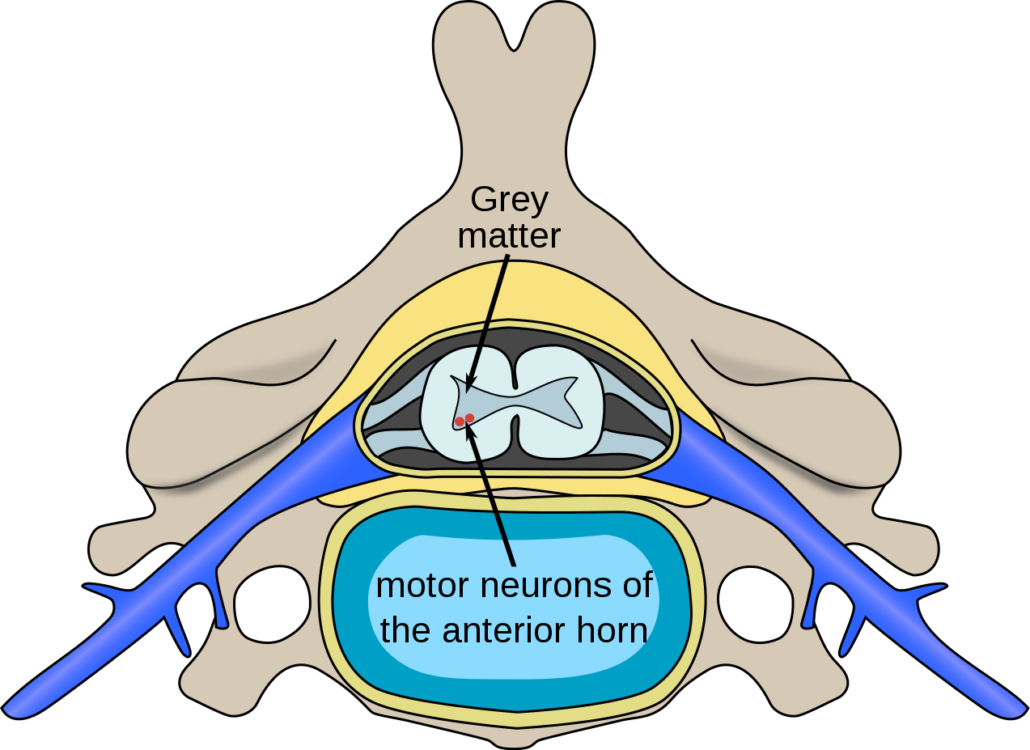
Spinal Muscular Atrophy (SMA) is an inherited, genetic disease with only one approved treatment. The disease is caused by a mutation in a gene that encodes for an important protein involved in the survival of motor neurons, which control muscle function. When this protein is deficient, muscles do not form properly, and/or die prematurely. This disease affects infants, children, and even, to a lesser extent, adults. Many of these babies die before their first birthday, and many of the children cannot even sit without help.


 Unsplash+
Unsplash+
