Hansa Biopharma’s imlifidase advances to Phase III testing
The US Food & Drug Administration has given the green light for Phase III testing of Hansa Biopharma's imlifidase in anti-GBM disease.
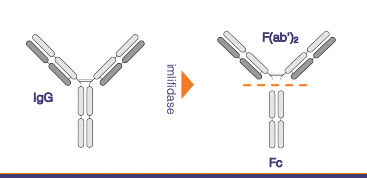
According to Lund-based Hansa Biopharma AB the study protocol has been greenlighted both by the FDA and the European Medicines Agency (EMA), so its pivotal Phase III study can commence this year. Phase II data suggested that deactivation of autoantibodies by imlifidase, an IgG-digesting enzyme that acts as an immunosupressant, could alter the course of the serious autoimmune disease anti-Glomerular Basement Membrane (anti-GBM) disease.
The pivotal Phase III study will enroll 50 patients with anti-GBM disease across the U.S. and Europe. Patients will be randomised 1:1 to receive either Standard of Care or imlifidase plus Standard of Care. The planned primary endpoint of the study is renal function by means of eGFR at six months. Patients will also be evaluated for other parameters related to kidney function during a six-month follow-up period.
According to data from an investigator-initiated Phase II study by Professor Mårten Segelmark (NCT03157037) kidney function at 6 months was significantly better than in previously published cohorts, without any safety concerns. Of the 15 patients included, 10 were dependent on dialysis at enrollment. At 6 months, a total of 67% (N=10) of the included patients were dialysis independent,versus a historical control cohort, (18%). All patients that were dialysis-independent at baseline remained so during the study. This positive outcome has been recognized for its significance, as it suggests that deactivation of autoantibodies by imlifidase could alter the course of an autoimmune disease.
Imlifidase was granted Orphan Drug Designation in anti-GBM disease by both the FDA and the European Commission in 2018.


 BioDlink
BioDlink
 Unsplash+
Unsplash+