Gut bacteria seed autoimmunity-inducers
A bacterium of the gut microbiome seems to spark an autoimmune disease in the kidneys by leaking antigens that trigger the immune system.
A unique mechanism, reported by an Irish-French research team underscores an emerging theme in microbiome research: that disruptions to gut bacteria can have far-reaching effects on the immune system elsewhere in the body. Autoimmune disorders typically manifest when immune cells mistakenly recognise the body’s own antigens as foreign.
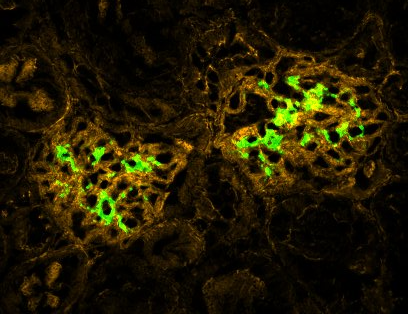
Now Patrick Gleeson from University College Cork and Renato Monteiro from Centre de Recherche sur l’Inflammation in Paris report that autoimmune conditions can be shaped by the body’s microbiome, although a direct causal link is yet missing. Examining the relationship between the gut microbiome and the emergence of IgA nephropathy, the scientists found that the gut microbiomes of 33 patients with the disorder were unusually skewed toward bacterial species that degrade mucin, including Akkermansia muciniphila.
In culture and in mice, this bacterium modified IgA1 and converted it into a deglycosylated form associated with IgA nephropathy. This process created new antigens that crossed the lining of the intestines and entered the blood, eventually settling into the glomeruli region of the kidneys. Supporting this model, the scientists found that mice expressing human IgA1 ended up developing IgA nephropathy after being colonised with A. muciniphila.
Additionally, the team also found that antimicrobial peptides called alpha-defensins suppressed the growth of A. muciniphila in culture, but that these protective effects seemed to be lost in stool samples from patients. These results support a previously unknown mechanism of disease whereby a secreted human glycoprotein is modified by bacteria in the intestinal lumen before reintegrating into the host, where it is no longer recognisable as self, Gleeson et al. conclude.



 Microbiotica
Microbiotica lkov - adobe stock photo
lkov - adobe stock photo