Evotec SE cashes in €20m milestone
Evotec SE has reached a predefined milestone worth €20m in the CRO's partnership with Bristol Myers Squibb (BMS).
The partnership initiated in 2016 uses Evotec’s iPSC platform to create predictive patient-derived cell-based disease models that mimic the pathology of neurologic diseases better than small animal models. Induced pluripotent stem cells (iPSCs) can devide indefinetely and differentiate in every cell type of the human body if cultured with the proper mix of growth and differentiation factors. In March 2023, BMS upgraded the partnership by adding another cell type to it, and extended the ongoing partnership for another eight years. A target-based programme is now ready for Phase II clinical development which triggered the €20m milestone payment to Evotec.
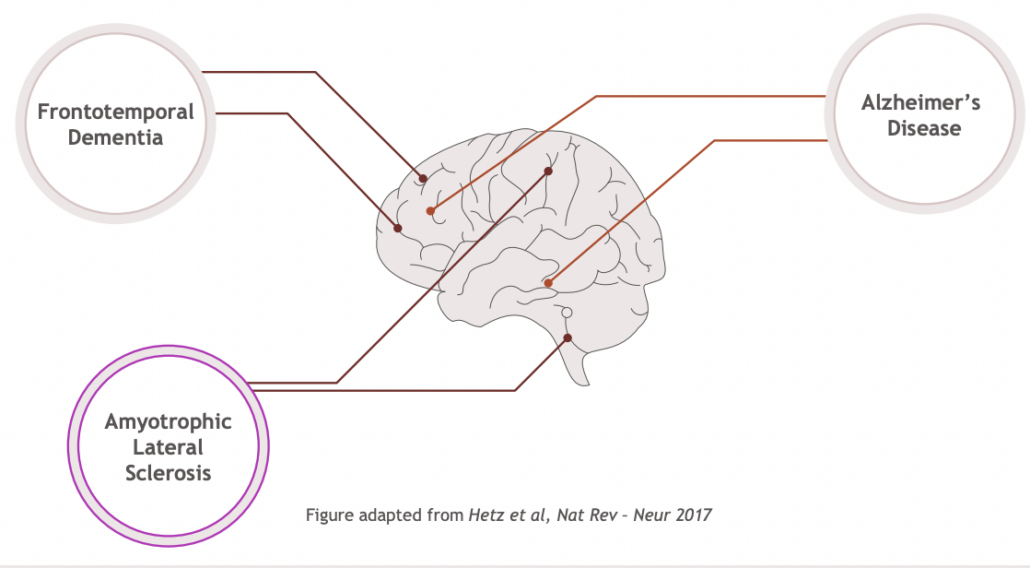
Currently, approved drugs provide only short-term treatment of symptoms in neurodegenerative diseases and there is a urgent need for therapeutic modalities that slow or prevent disease progression. BMS in-licensed a first programme, EVT8683, as BMS-986419 in September 2021, which is currently tested as treatment for two blockbuster indications, Alzheimer’s disease and amyothrophic lateral sclerosis, and has potential as a treatment for frontotemporal dementia. Following a successful Phase I trial that included multiple ascending doses of BMS-986419, Bristol Myers Squibb announced that a Phase II trial for BMS-986419 is planned for H2/2024.
BMS-986419 targets the eIF2 complex a master regulator of the integrated stress response (ISR), which is permanently overactivated in a wide range of brain disorders. By binding and agonising eIF2B, the small molecule reversed the causative mechanism underlying the cognitive deficits and neurodegeneration restoring normal ISR function, protein clearance and cellular homeostasis.
With BMS-986446, BMS has another Alzheimer drug in Phase II proof-of-concept testing that binds to MTBR-Tau 243 and has been demonstrated to correlate well with tau accumulation as measured by TauPET imaging and cognitive impairment. In its focus areas oncology, cardiovascular and neurologic disorders, BMS expects to increase the number of first-in-class compounds from 6 to 12.




 White House
White House