CAR-T: Novartis prices CTL019 at US$475,000
Swiss Novartis AG has received US market authorisation for its first-in-class CAR-T cell therapy Kymriah (tisagenlecleucel-T). As severe cytokine storms seemed to be generally linked to CAR-T therapies such as Kymriah, the FDA also authorised Roche's anti-IL-6R antibody Actemra (tocilizumab) as first-in-class treatment to manage them.
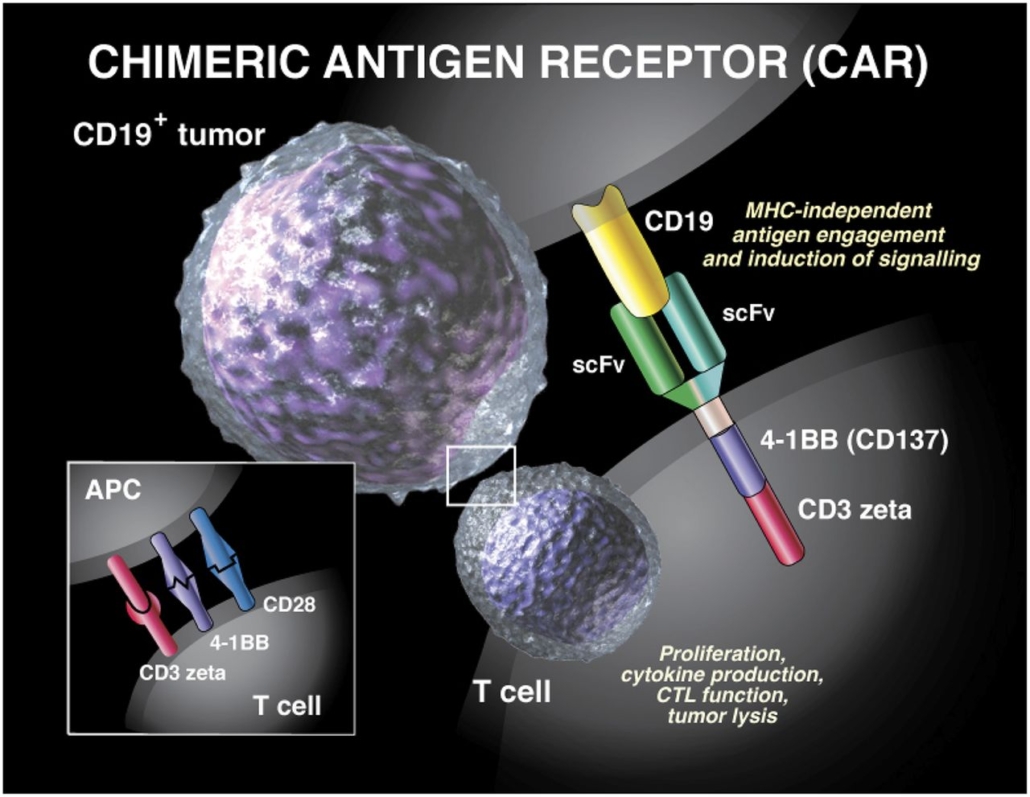
Novartis, which received unanimous backing from the FDA ODAC committee for its CAR-T cell therapy in July to treat young patients with acute lymphoblastic leukemia (ALL), priced Kymriah at US$475,000 per patient, making it one of the most expensive treatments in the world. The pharma major also agreed with the CMS (US Centers for Medicare & Medicaid Services) that patients who do not respond to its therapy won’t be charged. Kymriah consists of autologous T cells which were engineered ex vivo to target the CD19 antigen on B lymphocytes. As the CD19 antibody is linked to a immunostimulatory CD3zeta/CD137 signalling domain, T cells kill B lymphocytes upon binding.
The Novartis CAR-T approval is a significant progress for patients and patient communities of the diseases that late-stage clinical CAR-T therapies are targeting, commented the International Society for Cellular Therapy. Besides ALL, Novartis is seeking approval for tisagenlecleucel-T in non-hodgkin lymphoma and diffuse large B cell lymphoma. In September, the FDA will decide over market authorisation of Kite Pharma’s CAR-T cell therapy axicabtagene ciloleucel targeting the CD19 antigen on B lymphocytes.Most recently, the company has been subject to a US$11.9bn take-over bid by Gilead Sciences.
FDA approval for Novartis’ breakthrough therapy was based on the ELIANA trial in which tisagenlecleucel-T showed a remission rate of more than 80% in 63 ALL patients at three months. As up to now, four deaths caused by CAR-T induced cytokine release syndrome (CRS) occured in clinical trails evaluating different CAR-T therapies. Since additional 44% of patients who were enroled in the ELIANA trial showed grade 3 and 4 CRS, the FDA also expanded the label of Roche/Genentech’s RA antibody tocilizumab (Actemra) to treat CAR T cell-induced CRS. Actemra helped 69% of patients to resolve CRS 14 days after they received the monoclonal antibody. Until today, there has never been an FDA-approved treatment to manage severe cytokine release syndrome associated with CAR T cell therapy, which is marked by a rapid onset and can cause life-threatening complications, said Sandra Horning, MD, Chief Medical Officer and Head of Global Product Development at Roche. Today’s approval of Actemra/RoActemra for CRS provides physicians with an important tool to help manage this potentially life-threatening side effect.
According to the FDA, Kymria must be co-administered with Roche’s immunosuppressive antibody therapy for management of CRS. However, Actemra could not resolve neurotoxicity of tisagenlecleucel-T treatment, which occured in 44% of patients in the ELIANA trial. As manufacturing of Novartis’ CAR-T cell therapy is lenghty, complicated and only partly reproducible, the 20-25 medical centre expected by Novaertis set to offer the therapy must receive certification before.




