British PrecisionLife Ltd inks partnership with Metrodora Institute
British PrecisionLife Ltd and the Metrodora Institute have announced to launch clinical trials to improve the diagnosis and therapy of long COVID-related ME/CFS.
The Oxford-based Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) expert PrecisionLife Ltd and US-based clinical specialist Metrodora Institute (Salt Lake City) announced they will recruit up to 1,000 patients for biomarker-guided clinical trials expected to evaluate new diagnosis and treatment options for ME/CFS, long COVID, and related disorders.
The partners have developed genotypic diagnostic tests that are now being evaluated for their ability to provide clinicians with an accurate view of a patient’s risk of a specific disease. Ultimately, using the molecular profile of their underlying disease mechanisms, these tests will also be used to recruit cohorts to evaluate the effectiveness of drug repurposing candidates targeting specific disease mechanisms to improve patient outcomes.
The partnership builds on PrecisionLife’s unique insights into the mechanisms driving ME/CFS and Long COVID, Metrodora’s clinical and research expertise, and collaborations with patient charities including Action for ME and the world’s largest ME/CFS study, DecodeME. Recruitment for the trial will open in June and will be accessible through Metrodora’s research platform.
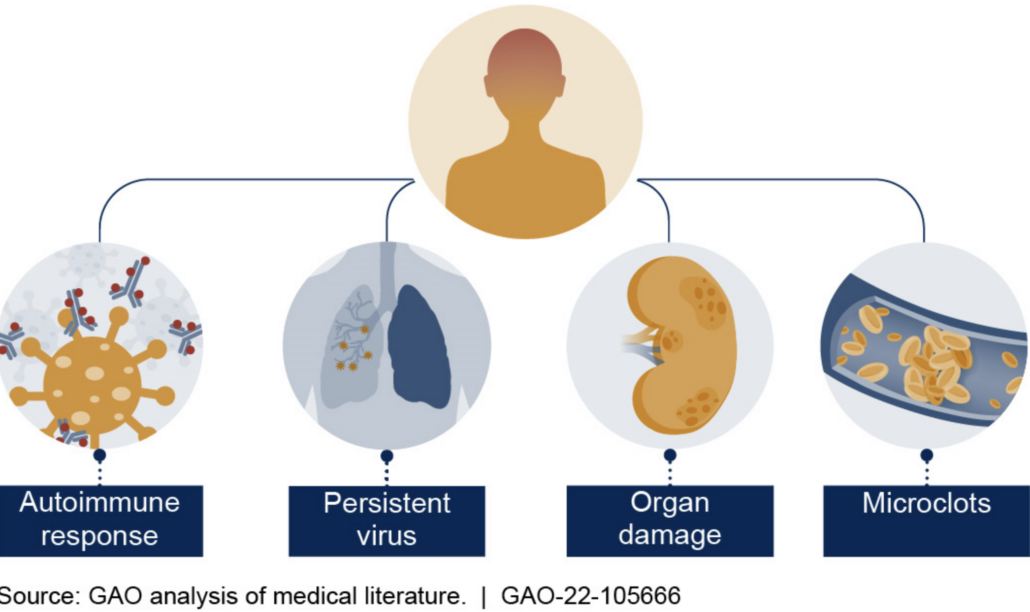
In mid-January, high-throughput proteomic analyses of the blood serum of 113 patients who had either fully recovered from COVID-19 or developed long-Covid gave hope for improved future diagnosis and treatment (doi: 10.1126/science.adg7942). First author Carlo Cervia-Hasler and colleagues from the University Hospital Zurich measured the serum levels of 6,596 human proteins in infected and healthy individuals immediately, six and twelve months after infection. Using high-throughput proteome analysis methods, they identified changes in serum proteins as a probable cause. The results promise to improve biomarker-based long-Covid diagnosis and at the same time provide new drug targets. The biomarkers indicate impaired activation of the complement system, altered coagulation and tissue damage, which in turn suggests persistent thromboinflammatory reactions in which monocyte-platelet aggregates show elevated levels. Dysregulation of complement proteins could therefore contribute to the thromboinflammation associated with long-COVID. To date, the use of coagulation and complement inhibitors has led to mixed results in the treatment of long-COVID
According to first author Dr Carlo Cervia-Hasler, ‘we looked at 40 patients who still had long-COVID symptoms after six months. We compared the blood serum of these patients longitudinally with the blood serum of patients who had become symptom-free again after infection and with the blood of the 39 non-infected controls. We published the results in Science and looked at the 40 patients who still had long-COVID symptoms after six months. We compared the blood serum of these patients longitudinally with the blood serum of patients who had become symptom-free again after infection and with the blood of the 39 non-infected controls. We then looked at what had changed the most.’
Mechanistically, the Swiss are as far as one can research in humans. ‘We saw in the blood that the factor C5b-C7 complex – i.e. late processes in the complement cascade – was poorly expressed, i.e. low, while the early proteins of the complement cascade, especially the soluble C5b-C6 complex, were elevated. As soon as C7 binds to the other complement proteins, it can enter the cell and form the terminal C5b-C9 complement complex, i.e. the cell-destroying pore. Our interpretation is that when C7 is deep, it disappears somewhere. This can be explained, for example, by an increased membrane insertion of the terminal complement complex triggered by the C7 complexes, which results in tissue damage. The tissue damage could possibly be the reason for the complement activation. Consistent with this, long-COVID patients showed increased markers of tissue injury in the blood, but also a thromboinflammatory signature characterised by markers of endothelial activation, such as von Willebrand factor (vWF), and red blood cell lysis. Low antithrombin III levels in long-COVID patients were associated with evidence of increased cleavage by thrombin, which may be partly responsible for the formation of the terminal complement complex. In addition, long COVID patients showed increased platelet activation markers and monocyte-platelet aggregates after six months, especially in cases where long-COVID lasted a year or longer. These patients also showed signs of antibody-mediated activation of the classical complement pathway, which was associated with elevated anti-cytomegalovirus, human herpesvirus 5, and anti-epstein-barr virus immunoglobulin G antibody levels,’ said Cervia-Hasler, who hopes the work will inform interested drug developers about useful targets.


 adobe stock photos - deemerwha
adobe stock photos - deemerwha stock.adobe.photo.com/Popelniushka
stock.adobe.photo.com/Popelniushka  Boehringer Ingelheim
Boehringer Ingelheim