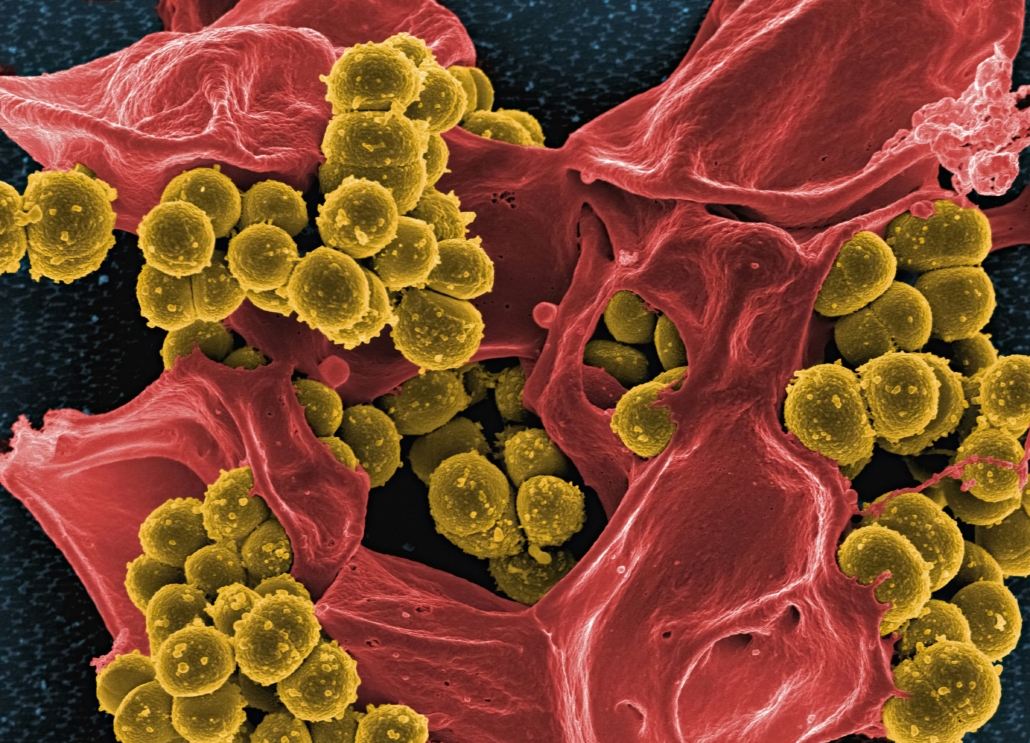
Basilea strikes up to $48.5m antifungal deal with U.S. biotech Prokaryotics
Swiss anti-infectives specialist Basilea Pharmaceutica has entered a research collaboration with U.S.-based Prokaryotics to develop a novel broad-spectrum antifungal aimed at treating severe invasive fungal infections, the companies announced on January 7th 2026.
Addressing a persistent treatment gap
The partnership targets a long-standing gap in infectious disease medicine: new antifungal therapies with mechanisms distinct from existing drugs. The program focuses on invasive infections caused by Candidaspecies, Aspergillus molds and a range of rare molds that disproportionately affect immunocompromised patients and are associated with high mortality.
Under the agreement, the companies will jointly advance a portfolio of antifungal molecules with a novel mode of action. Once a clinical candidate is selected, Basilea will take over responsibility for clinical development and global commercialization under an exclusive license.
“There remains a significant unmet medical need for safe, effective, and easy to administer antifungals, with activity against priority pathogens,” said Laurenz Kellenberger, chief scientific officer of Basilea, in a statement. He added that the collaboration reflects the company’s broader commitment to anti-infective research and development.
Deal structure and development pathway
Financial terms include an undisclosed upfront payment and near-term milestones from Basilea to Prokaryotics. The U.S. biotech could receive up to $48.5 million in development, regulatory and commercial milestone payments, as well as tiered low single-digit royalties on global net sales if the product reaches the market.
The collaboration is structured so that early discovery and preclinical efforts are shared, while later-stage development and commercialization would be fully led by Basilea. This approach reflects a growing trend in anti-infectives, where smaller biotechs partner with companies that have established hospital-focused development and regulatory expertise.
“There is a huge medical need for new antifungal agents with novel modes of action distinct from current clinically used drugs,” said Terry Roemer, chief scientific officer of Prokaryotics. He said the partnership aligns the company’s discovery capabilities with Basilea’s experience in advancing novel drugs to the market.
The rising burden of invasive fungal infections
Invasive fungal infections remain a major clinical challenge, particularly in hospital settings. Invasive candidiasis and candidemia are among the most common bloodstream infections in intensive care units, while invasive aspergillosis and infections caused by rare molds pose life-threatening risks to patients with hematologic cancers, organ transplants or other forms of immune suppression. Despite available therapies, outcomes remain poor, driven by delayed diagnosis, limited treatment options and emerging resistance.
Prokaryotics brings to the collaboration expertise in anti-infective discovery and medicinal chemistry, with a focus on targeting fundamental processes in microbial cell wall and cell envelope biology. The company was founded around antibiotic assets originally licensed from Merck & Co. and has since expanded its pipeline to include both antibacterial and antifungal programs.
Basilea, founded in 2000 and headquartered in Allschwil, Switzerland, has positioned itself as a specialist in severe bacterial and fungal infections. The company currently markets Cresemba for invasive fungal infections and Zevtera for serious bacterial infections.
The new collaboration adds to Basilea’s preclinical pipeline and underscores continued industry interest in addressing invasive fungal disease at a time when investment in anti-infectives remains limited despite growing clinical need.


 Genentech Corp. / Roche
Genentech Corp. / Roche Novo Nordisk
Novo Nordisk Resalis/Vimeo
Resalis/Vimeo