Amal bags €21.2m financing
Swiss Amal Therapeutics S.A. (Geneva) has raised €21.2m in a series B follow-up financing.
All existing investors – Boehringer Ingelheim Venture Fund, BioMedPartners and Helsinn Investment Fund, VI Partners, High-Tech Gründerfonds, and Schroder Adveq – participated in the follow up round to a €8m round closed in September 2017.
Amal Therapeutics said it will use the proceeds to start Phase I testing with its therapeutic cancer vaccine ATP128 next summer. The goal is to provide proof-of-concept in patients with colorectal cancer, a €9.6bn market opportunity. The company added that it will use its Kisima cancer antigen delivery technology to add further therapeutic vaccine candidates in different cancer indications to its development pipeline. A candiate vaccine to treat glioblastoma has been filed for patent.
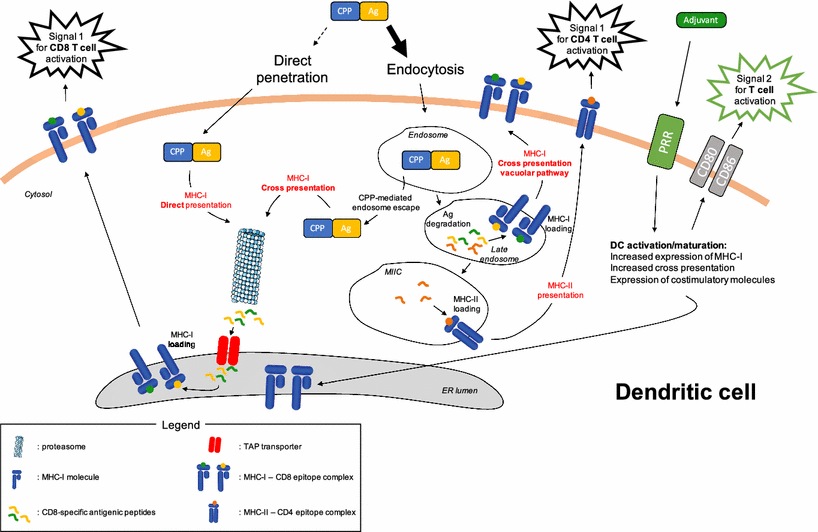
The 2012 spin-out company from University of Geneva uses cell penetrating protein domains (CPP) to deliver an TLR peptide agonist as adjuvant fused to cancer antigens into antigen presenting cells, which trigger CD4- and CD8-mediated immune responses through MHCI and MHCII antigen presentation.
For delivery, the company makes use of a 42 residue fragment (Z12) of the ZEBRA protein transduction domain, which can deliver multi-epitopic cargoes (Z12-Multi-E) into different processing compartments of dendritic cells.
Usually, protein vaccines primarily activate helper T cells, because the antigens are mostly processed and presented on MHC class II molecules. To improve MHC class I molecule presentation, Amal Therapeutics uses the proprietary ZEBRA peptide that promotes cross-presentation on MHC class I molecules and MHC class II presentation.


 Unsplash+
Unsplash+
 Bayer AG
Bayer AG