Ablynx takes Phase III hurdle in acquired TTP
Belgian nanobody developer Ablynx NV's nanobody caplacizumab has met relevant endpoints in the pivotal Hercules Phase III study in patients with thrombotic thrombocytopenic purpura (aTTP), paving the way to market authorisation in Europe and the US.
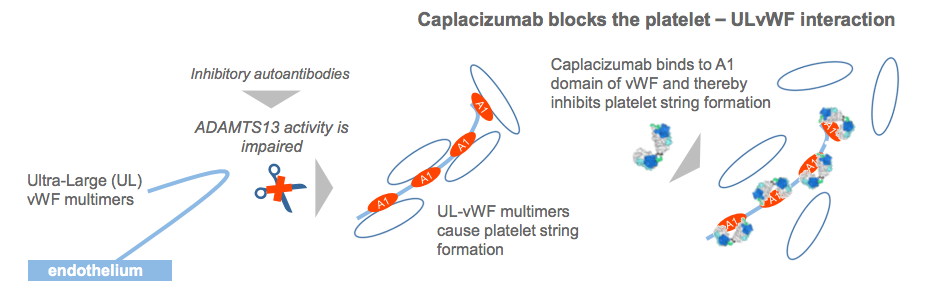
In July, caplacizumab received FDA fast track status for treating thrombotic thrombocytopenic purpura (aTTP), an orphan blood disease affecting 7,500 US Americans per year. Now, Ablynx (Ghent) announced that the bivalent antibody, which targets the von Willebrand factor (vWF) in order to prevent formation of platelet micro-clots that cause the severe thrombocytopenia, tissue ischemia and organ dysfunction in aTTP, met the primary and certain secondary endpoints in the pivotal Hercules Phase III study, which enroled 145 patients. Despite the current standard-of-care treatment consisting of PEX and immunosuppression, episodes of aTTP are still associated with a mortality rate of up to 20%, with most deaths occurring within 30 days of diagnosis
Treatment with caplacizumab in addition to standard-of-care resulted in a statistically significant reduction in time to platelet count response (p<0.01), the primary endpoint of the study and a measure of prevention of further microvascular thrombosis. Patients on caplacizumab were 1.5 times more likely to achieve platelet count response at any given time point, compared to patients treated with placebo.
The Phase III HERCULES study also met the first two key secondary endpoints. Treatment with caplacizumab resulted in a 74% reduction in the percentage of patients with aTTP-related death, recurrence of aTTP, or at least one major thromboembolic event during study drug treatment (p<0.0001), with recurrences being the driver for achievement of this endpoint. In addition, the proportion of patients with a recurrence of aTTP in the overall study period (including the 28 day follow-up after discontinuation of study drug treatment) was 67% lower in the caplacizumab arm compared to the placebo arm (p<0.001), demonstrating the durability of the treatment effect.
Analysis of the third key secondary endpoint showed that no patients treated with caplacizumab were refractory to treatment compared to three patients treated with placebo (p=0.057). The analysis of the fourth key secondary endpoint did not achieve significancy level but showed a trend to faster normalisation of the organ damage markers (lactate dehydrogenase, cardiac troponin I and serum creatinine) in patients treated with caplacizumab.
The number and nature of treatment-emergent adverse events (TEAEs) were similar between the treatment groups. Serious TEAEs were more common in the placebo group, driven by the percentage of patients experiencing a recurrence of aTTP. The percentage of subjects with any bleeding-related TEAE was higher in the caplacizumab treatment group than in the placebo treatment group (66.2% vs. 49.3%). Most bleeding-related TEAEs were mild or moderate in severity. There were three deaths in the placebo group and none in the caplacizumab group during the study drug treatment period. One patient in the caplacizumab group died in the follow-up period after completing the study drug treatment and this was assessed by the investigator not to be related to study drug.
A three-year follow-up study (NCT02878603) of patients who have completed the Hercules study is in progress and will further evaluate the long-term safety and efficacy of caplacizumab and repeated use of caplacizumab, as well as characterising the long-term impact of aTTP.
In February 2017, based on the Phase II Titan study results, a Marketing Authorisation Application (MAA) was submitted to the European Medicines Agency (EMA) for approval of caplacizumab in aTTP. Ablynx expects the results from the Phase III HERCULES study to support the MAA, as well as a planned Biologics License Application (BLA) filing in the United States in 2018. If approved by regulatory authorities, caplacizumab would be the first therapeutic specifically indicated for the treatment of aTTP. Ablynx expects peak sales of more of €400M in the indication.
aTTP is a rare, acute, life-threatening, autoimmune blood clotting disorder. It is caused by impaired activity of the ADAMTS13 enzyme, leaving ULvWF molecules uncleaved. These ULvWF molecules spontaneously bind to blood platelets, resulting in severe thrombocytopenia and clot formation in small blood vessels throughout the body, leading to ischemia and widespread organ damage.



 Unsplash+
Unsplash+