The optically pumped semiconductor laser (OPSL) is an ideal light source for structured illumination microscopy (SIM), a laser fluorescence technique that can deliver unique information in drug discovery.
ADVERTISEMENT
The rapid growth of active pharmaceutical ingredients (APIs) has led to more biotech companies enlisting the support of strategic partners to navigate the regulatory environment and bring their innovative products to market.
The 12th edition of the European Biotechnology Science & Industry Guide once more offers an interesting cross-section of the European biotech scene.
Building a sustainable future this is what drives us. We are passionate about making smart biotech materials part of everyday life. We believe nature offers better alternatives to existing materials with biofabricated technologies previously unimaginable – enabling our customers to make a true change.
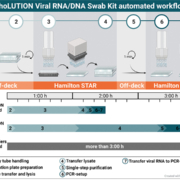
The SARS-CoV-2 virus global outbreak generated a need for fast and reliable large-scale testing. One of the main bottlenecks of the PCR test is the laborious and time-consuming molecular method for viral genetic material extraction. BioEcho Life Sciences, together with Hamilton and the German diagnostics laboratory Labor Dr. Fenner und Kollegen’ developed an automated workflow for 96- and 48-well plate formats.
ADCs have huge potential in treating challenging diseases such as cancer. However, it is essential that all necessary equipment, steps, and precautions are in place to en¬sure their safe handling.
Sino Biological, a biotechnology company listed on the Shenzhen stock exchange subsidiary ChiNext (SZSE: 301047), which provides biological research reagents and related technical is pleased to announce a CRO services partnership with Ainnocence, Inc. of San Jose, California.
The life sciences location of Austria has gained considerable momentum since the founding of the very first Austrian biotech company Intercell at the turn of the millennium. Today the who’s who of the biotech sector relies on Austria, in particular on its capital city of Vienna.
Saxony has established itself as a dynamic life-sciences hub and provides an attractive environment for investors and start-ups The SaxoCell’ network and CMI major research centre are furthering innovations in cell and gene therapy Interdisciplinary co-operation offers huge growth opportunities for applications such as point-of-care diagnostics Leipzig as host of BIO-Europe 2022



 Lonza Group
Lonza Group