Azenta Inc has entered into an agreement to acquire B Medical Systems S.á r.l. and its subsidiaries for €410m.
ADVERTISEMENT
Sartorius Stedim Biotech has purchased the world’s largest human albumin producer Albumedix to broaden its product portfolio in animal-free cell media and to stabilise protein drugs.
As Genmab’s Hexabody tumour antibody platform is advancing, BioNTech has secured a licence in the field of immunotherapy to co-develop antibodies on 50:50 base.
British antifungal developer F2G Ltd has announced a $70mn financing round to push development of its late-stage compound olorofilm.
Sosei Heptares and AbbVie have inked a multi-target licence option drug discovery agreement in neurology.
Researchers have presented a novel method to promote biofilm formation and thus to increase efficiency of biocatalysis in biotech production.
InflaRx plans to apply for Emergency Use Authorization from the US FDA in Q3/2022 for vilobelimab, the company’s treatment for critically ill COVID-19 patients.
Through a $325m SPAC transaction with Frazier Lifesciences Acquisition Corporation, NewAmsterdam Pharma Holding B.V. will go public on Nasdaq. The newly formed holding company, NewAmsterdam Pharma Company N.V. will be led by Michael Davidson, M.D., CEO. The late clinical-stage company develops small molecule therapies for some cardiometabolic diseases.
The approximately $235m will come from an upsized and oversubscribed PIPE at $10.00 per share plus funds held in FLAC’s trust account following any redemptions. The PIPE was co-led by Frazier Healthcare Partners and Bain Capital Life Sciences, and includes new investors RA Capital Management, GMT Capital, Medicxi Ventures, Panacea Venture and other institutional investors, in addition to existing NewAmsterdam shareholders Forbion, LSP Dementia Fund and Morningside Ventures. The proceeds from this transaction, combined with an upfront payment of $123m (€115m) from the company’s recent licensing agreement with the Menarini Group and NewAmsterdam’s existing cash and cash equivalents, would bring NewAmsterdam’s total pro forma cash balance to at least $470m, which the Company expects to fund operations through 2026, beyond the readout of NewAmsterdam’s Phase III PREVAIL CVOT study and two other pivotal Phase III studies. The transaction is expected to close in the second half of 2022
Cardiovascular disease is the number one cause of death in western countries, with high LDL levels being the chief culprit responsible for adverse outcomes and two-thirds of patients not reaching LDL goals despite the wide availability of statins. New Amsterdam’s Phase III oral therapy has been demonstrated to confer an additional 51% of LDL-lowering on top of high dose statins Proceeds from the transaction are expected to provide NewAmsterdam with the capital needed to further develop obicetrapib through several value-creating clinical and regulatory milestones, including Phase III testing of the drug in adults with heterozygous familial hypercholesterolemia and/or established atherosclerotic cardiovascular disease who require additional lowering of low?density lipoprotein cholesterol on top of high intensity statins; Phase III trials in adults with HeFH, whose LDL-C is not adequately controlled despite being on maximally tolerated lipid-modifying therapies. The goal is to market obicetrapib in the United States, Europe, Japan and China.
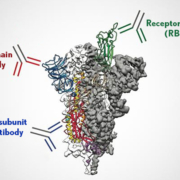
A nasal antibody cocktail binding to the conserved S2 stalk region of SARS-COV-2 might end the corona pandemic.
This new investment will support the growth of the Mourenx site and sustain the growing demand for production of active pharmaceutical ingredients (APIs), particularly the highly potent drugs (HPAPIs) used to treat cancer.