Breaking news from biotechnology and the German stock market are in the focus of the new issue of BIOTECH Insight, the newsletter of Deutsche Börse AG.
ADVERTISEMENT
Carbios SAS has announced a capital increase of around €122m that may grow up to €141m.
This year’s Veeva R&D and Quality Summit in Madrid, accentuated the current trends and needs of the pharma and life sciences industries.
Santhera Pharmaceuticals Holding has exclusively granted the rights to commercialise its Duchenne muscular dystrophy drug vamorolone in North America to Catalyst Pharmaceuticals Inc.
BioSenic SA has suspended its Phase II proof-of-concept trial on fracture healing with its allogeneic cell therapy ALLOB.
Swedish personalised immunotherapy specialist Neogap Therapeutics AB has acquired the remaining shares of its subsidiary, TCER Oncology AB through two new shareholders.
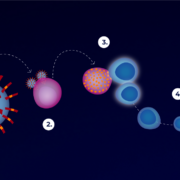
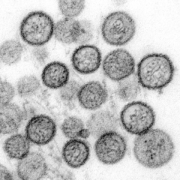
An international research team headed by Pablo Guardado-Calvo at Institut Pasteur Paris has demonstrated evaluated antibody efficacy against lethal hantaviruses.
Syncona Ltd had spun-out the ophtalmology specialist Beacon Therapeutics Holdings Ltd with a £96m Series A financing.
Roche AG has announced positive results from the non-blinded Phase III COMMODORE 1 and 2 trials at the EHA Hybrid Congress in Frankfurt, Germany.
The world 3rd largest gene and cell therapy (CGT) cluster, the UK CGT Catapult has expanded its operations opening a subsidiary in Edinburgh.