The US Roche unit Genentech Inc has signed a US$400 million licence deal with British NativeMS specialist OMass Therapeutics Ltd. Under the exclusive collaboration and licence agreement, Genentech intends to commercialise therapies for inflammatory bowel disease.
ADVERTISEMENT
Sanofi SA has received FDA marketing approval for Wayrilz (rilzabrutinib). Unlike three existing causal treatments for immune thrombocytopenia (ITP), the oral drug inhibits both processes that drive the autoimmune disease. Analysts therefore predict peak sales twice as high as those of previously approved ITP drugs.
British company Sitala Bio Ltd, founded in 2021 by NLRP3 inflammasome expert Matt Cooper, is paying US$670m and giving up to 10% of its shares to Chinese company Shanghai Fosun Pharmaceutical Group Co. Ltd. in exchange for the development and marketing rights to the low-molecular-weight inflammation blocker FXS6837.
Eli Lilly, the US market leader in the lucrative diabetes and obesity space, is now challenging its competitor Novo Nordisk in the area of oral GLP-1 receptor agonists. Lilly’s orforglipron has met the primary endpoint of its registration study and is set to be submitted for approval in the United States.
Norway-based Calluna Pharma A/S has initiated patient recruitment in late August for its Phase II AURORA trial investigating safety and efficacy of CAL101, a monoclonal antibody with unique mode of action to cure idiopathic pulmonary fibrosis.
Following the commercial launch of BioArctic AB’s Alzheimer’s antibody lecanemab, the Swedish company has announced a new deal: it will receive an upfront payment of US$30m, granting the Swiss company access to BioArctic’s BrainTransporter platform to transport therapeutics for neurodegeneration across the blood-brain barrier
Swedish BioArctic AB’s partner Eisai Pharma has started the European roll-out of their Alzheimer’s antibody lecanemab in Austria that will be followed by the German launch on September 1, 2025.
The US and EU have confirmed a trade agreement involving US import tariffs of 15% on prescription medicines. Generics manufacturers will face a maximum tariff of 2.5%.
Germany’s Mallia Aesthetics GmbH has announced the achievement of key regulatory milestones in preparation for the market launch of its sCD83-based hair growth stimulant, MAL-838, later this year.
Novo Nordisk has entered a collaboration with research institute BioMed X in an effort to regain market position lost to leading competitors in diabetes and obesity treatment. The partnership is focused on improving the low bioavailability of orally administered peptide-based medicines.


 adobe stock photos - Sundry Photography
adobe stock photos - Sundry Photography  adobe stock photos - JHVEPhoto
adobe stock photos - JHVEPhoto Forbion
Forbion Adobe stock photos - Jim
Adobe stock photos - Jim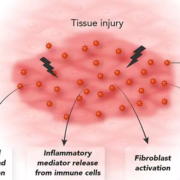 Calluna Pharma A/S
Calluna Pharma A/S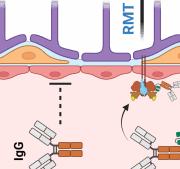 BioArctic AB
BioArctic AB Esai Pharma
Esai Pharma EFPIA
EFPIA![mode-of-action details[73] Mode of action of sCD-83](https://european-biotechnology.com/wp-content/uploads/2025/08/mode-of-action-details73-180x180.png) Mallia
Mallia BioMed X
BioMed X