Researchers have applied a self-learning algorithm to distinguish the almost 100 brain cancer types through their methylation pattern in a routine diagnostic setting. The method eliminates inter-pathologist variation in histopathological diagnosis and resulted in a change of diagnoses in up to 12% of prospective cases.
ADVERTISEMENT
The tremendous success of last year’s BIO Europe Spring in Barcelona was hard to beat. But the follow-up this year in Amsterdam organised by EBD Group together with Health Holland crystallised as a worthy successor.
German MYR Pharma has completed a proof of concept Phase IIb trial with its first-in-class hepatitis D entry inhibitor Myrcludex B, which is developed under the European Medicines Agency’s PRIME scheme.
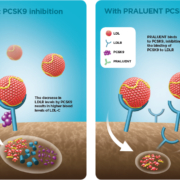
Alirocumab, Sanofi’ s/Regeneron’s PCSK9 inhibitor, in combination with statins has met the endpoint of reducing the number of major adverse cardiovascular events (MACE) over statin monotherapy in 18,924 high-risk patients.
Researchers at Medimmune, AstraZeneca’s biologics arm, have identified a mechanism that drives the autoimmune disease systemic sclerosis.
Evotec and Sanofi want to create an Evotec-led infectious disease open innovation R&D platform at Sanofi’s antiinfectives hub in Lyon. Talks are expected to close in H1/2018 with an option agreement.
Under an exclusive licence option agreement, Heidelberg Pharma will develop ATACs (alpha-amanitin antibody conjugates) for Magenta Therapeutics Inc. across several targeted conditioning programmes for bone marrow transplants.
Investments into new drugs to tackle antimicrobial resistance (AMR) are set to boost the participation of SMEs. At the 11th Berlin Conference on Life Sciences, two new financing initiatives, which focus on innovations developed by SMEs, were presented to 200+ AMR stakeholders.
Swiss Santhera Pharmaceuticals EU-approved LHON treatment Raxone (idebenone) has failed to show clinical efficacy in another indication: After US and EU regulators rejected an extension of market approval in Duchenne muscular dystrophy last September, an NIH-sponsored trail now showed no difference to placebo treatment in patients with primary progressive multiple sclerosis (PPMS).