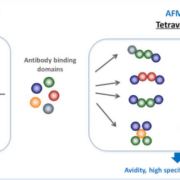
Roche’s US arm Genentech has licenced Affimed NV’s ROCK platform to add multivalent antibodies, which could activate the innate immune system, to its cancer pipeline.
ADVERTISEMENT
The British Heart Foundation (BHF) will tender £30m (€33.2m) to tackle the suffering and devastation caused by heart and circulatory diseases.
Orphan drug leader Shire plc (Dublin), has received the FDA’s approval for its antibody lanadelumab-flyo, which prevents hereditary angioedema (HAE) attacks.
Blocking the extracellular matrix (ECM) enzyme leukotriene A4 hydrolase (LTA4H) dampens inflammation but promotes changes in the airways of mice with allergic asthma due to accumulation of the ECM degradation product PGP.
Novo Nordisk and Evotec have joined forces to find new drugs in the lucrative diabetes and obesity markets.
French Genkyotex SA has secured an €7.5m gross financing to expand the scope of its Phase II product GKT831 in Primary Biliary Cholangitis (PBC).
Roche AG’s ALK blocker Alecensa (alectinib) has been granted market approval by China National Drug Administration as first line NSCLC monotherapy in patients with ALK-positive non small cell lung cancer (NSCLC)
BioNTech AG has partnered with Pfizer to develop a mRNA-based flu vaccine.
EGFR agonists support the protective effect of Langerhans against UV-induced damage of skin cells, a hallmark of lupus erythematosus and other autoimmune and dermatologic conditions, a team of US and German researchers report.
A team of Scots, Belgian and Spanish researchers claim they have found a target to prevent failure of liver regeneration following intoxination or acute injury.