Drug Developers dealing with PD1-PDL1 checkpoint inhibitors must consider new safety problems associated with the hyped cancer meds.
ADVERTISEMENT
The European Investment Bank (EIB) has issued its first Sustainability Awareness Bond worth €500m to support investments into water projects – health and nutrition to follow.
Around 300 international AMR experts met at ESCMID-ASM drug development conference in Lisbon to discuss the latest progress in the field.
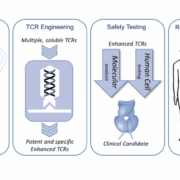
Oxford-based Adaptimmune Therapeutics plc has raised US$100m in a registered direct offering of American Depositary Shares (ADS).
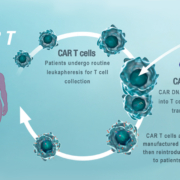
The NHS England announces it will reimburse Novartis’ CAR-T cell therapy Kymriah as treatment for children with B cell acute lymphoblastic leukemia (ALL) that are refractory, in relapse post-transplant or in second or later relapse.
Exosome drug delivery specialist Evox Therapeutics Ltd has closed a €37.1m (£35.5m) Series B financing round led by Redmile Group and significant contributions from GV (Google Ventures) and Cowen Healthcare Investments.
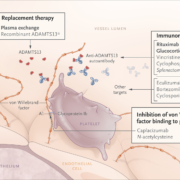
The EU approved Sanofi’s Cablivi (caplacizumab) as the first antibody therapeutic to treat acquired thrombotic thrombocytopenic purpura (aTTP) on Friday.
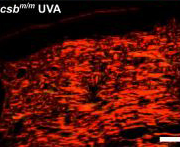
An FDA-approved cancer drug stops light sensitivity of the skin in Cockayne syndrome (CS), an ultra-rare hereditary disease characterised by premature aging, German researchers report.
Germany’s government announced that it will create an agency for breakthrough ("jump") innovation to foster technology transfer of risky research projects with high market potential into products.