German researchers have designed a modified Sleeping Beauty transposase that can be used for non-viral, stable and efficient gene insertions for gene and CAR-T therapies.
ADVERTISEMENT
Synbio specialist Sherlock Biosciences and next-generation lateral flow testing expert Mologic Ltd have joined forces to develop a no-instrument rapid testing platform detecting virtually every infectious pathogen in low resource settings.
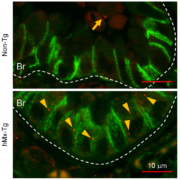
German researchers have shown that a protein protects against the development of liver fibrosis by shutting down inflammatory signalling in liver stellate cells of the liver.
Cell reprogramming specialist bit bio has expanded its management with renowned experts in the field.
Swiss biotech company BioVersys AG has received a €8m grant by US-based non-profit organisation CARB-X to develop novel anti-virulence antibiotics to treat severe bacterial infections.
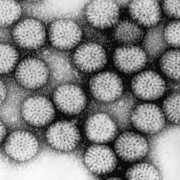
A team of Japanese and German researchers has found the protein that helps activate the inflammasome following viral invasion.
Researchers at University Lyon have found a potential repurposing application of two market-approved rotavirus vaccines.
Swiss Polyphor AG and researchers from the University of Zurich have presented the mechanism of action of a new class of antibiotics.
Zealand Pharma A/S has acquired Encycle Therapeutics Inc, including a peptide therapeutics platform and library.