BioNTech SE and its partner Fosun Pharma Co. Ltd have selected another COVID-19 vaccine candidate for Phase I testing in China than tested in global Phase III trials.
ADVERTISEMENT
Evotec SE has secured a strategic partnership with Secarna Pharmaceuticals GmbH to co-develop a LNA drug pipeline.
Rapid tests have received a lot of attention during the Coronavirus pandemic, but as the number of tests available has increased, so has the confusion around the different types and what they can deliver. What if there were laboratory quality tests that could produce a result in just a few minutes?
The European Commission has reserved 300 million doses from Sanofi SA’/GSK plc’s adjuvanted recombinant COVID-19 vaccine.
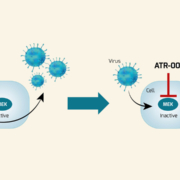
Viral pandemics are not a once-in-a-lifetime event but a constant threat looming over our heads. While the fight against SARS-CoV-2 is currently a top priority, we should not lose sight of what might come next. Broad-spectrum antiviral drugs like Atriva’s ATR-002, currently in Phase II clinical development for treating COVID-19, could be an effective measure in the fight against the current pandemic and future viral outbreaks.
Roche has reported the failure of its IL-6R antagonist tocilizumab in patients with COVID-19 putting the efficacy of IL-6 inhibition at question.
Italian start-up Enthera Pharmaceuticals has raised €28m to advance restorative therapy for type 1 diabetes and inflammatory bowel disease.
Oncolytic viruses, viral vectors for gene therapy and viral-vectored prophylactic vaccines, are new, innovative, and already successful approaches for the treatment of severe diseases. The production of these viruses requires extensive experience and specially-designed GMP clean rooms. Vibalogics offers both.
Swiss Polyphor AG has strengthened its financial flexibility with an equity-linked financing.
According to current information, the European Research Framework Programme "Horizon Europe" is likely to be among the indirect victims of the Corona pandemic: A large part of the funds earmarked will now go to the € 750 billion rescue fund.