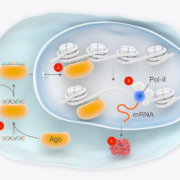
RNA therapeutics specialist MiNA Therapeutics plc has secured £23m in a Series A financing to push development its first-in-class small activating RNA (saRNA) candidates.
ADVERTISEMENT
Nine CEOs of COVID-19 vaccines have assured that safety of vaccinated individuals remains their top priority in development of the first COVID-19 vaccines.
The European Investment Bank has lend up to €10m to Polish microbial diagnostics specialist Scope Fluidics AS.
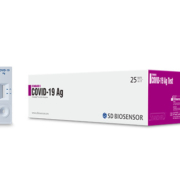
Roche has announced to launch a SARS-CoV-2 point-of-care antigen test this month co-developed with SD Biosensor Inc. (South Korea).
Unilever has announced to invest €1bn globally to eliminate fossil fuels as a source of carbon in its cleaning products by 2030.
With around 450 attendees, the five-day digital conference particularly focused on market conditions and financing. SMEs and global partners urged for more pull incentives.
Swiss BioVersys AG has secured a CHF19m Series B financing to push clinincal development of two antiinfectives against drug-resistant pathogens.
PPF-owned company SOTIO has acquired the rights to use the BOXR CAR-T platform and products from Cambridge-based Unum Therapeutics.
Polyphor AG has licenced its metastatic cancer drug balixafortide to Fosun Pharma Co Ltd to market the drug exclusively in China.
British fibrosis therapeutics start-up Engitix Ltd has entered a collaboration agreement with Takeda to develop anti-fibrotic therapies in liver diseases.