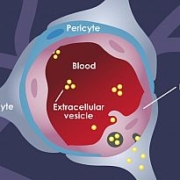
NeuVasQ Biotechnologies has raised €20m through a Series A investment to advance its technology platform that restores the integrity of the blood-brain barrier in acute neurological disorders.
ADVERTISEMENT
Coriolis Pharma will launch operations at its currently expanded ATMP development facilities by Q4/2021.
Company seeks new opportunities for use of mRNA therapeutics.
U.S. government to purchase at not-for-profit price 200 million doses in 2021 and 300 million in the first half of 2022.
Funding to accelerate clinical progression of Iksuda’s pipeline of new generation of antibody drug conjugates, targeting tumours with high unmet need.
Lonza will expand its production capacity of Moderna’s mRNA vaccine mRNA1273 at its manufacturing site in Geelen, the Netherlands.
The European Medicines Agency (EMA) has given the green light for vaccination of adolescents with Biontech’s COVID-19 vaccine Comirnaty (BNT162b2).
Senti Biosciences and Bayer subsidiary BlueRock Therapeutics form strategic partnership to combine iPSC and gene circuit-engineered cell therapy platforms.
German-US Anticalin specialist Pieris Pharmaceuticals has bagged $20m upfront for a strategic R&D and license agreement with Genentech, the US branch of Swiss Roche AG.
|
|
|
||
Life science associations are calling for ATMPs to be exempted from the strict EU GMO rules, as their different legal interpretations in EU member states are delaying applications for multicentre clinical trials.