Since the earliest attempts at genetic engineering, scientists have sought to influence cell behaviour and development through the introduction of foreign genetic material. Synthetic biologists are now taking the idea to a whole new plane with app-like DNA-based software programs that turn cells into diagnostic sensors, therapeutic entities or tissue generators.
ADVERTISEMENT
For decades, researchers have searched without success for a strategy to prevent malaria. Now, two teams of researchers present a solution that circumvents the problem of surface antigen hypervariability of Plasmodium. Their drug candidate blocks the pathogens’ release from blood cells, resulting in prevention of the life-threatening hot stages.
Spanish biotech sector:?European investors more and more often take a look at Southern European biotech companies. Spain, in particular, is now harvesting the fruits of long-term public support started a decade ago, which stimulated the establishment of a dynamic ecosystem. Today, foreign investments are at an all-time high and start-ups find a well-prepared basis for growth.
A complaint against the EMA’s handling of a referral procedure related to potential side effects of Sanofi/MSD’s HPV vaccine Gardasil and GSK’s HPV jab Cervarix, filed by the Nordic Cochrane Centre and physicians, has been rejected by the European Ombudsman.
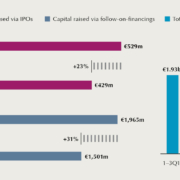
The European biotech stock market witnessed an increase in IPO and follow-on financing volumes in 2017. According to the new BIOCOM capital market report, published in November during BIO-Europe in Berlin, more than half the money was raised on the US Nasdaq. Within Europe, Euronext is still is the most attractive location – but Stockholm is catching up.
A series of high-level conferences held in autumn and winter focused on current challenges to implementing an industrial scale bioeconomy in Europe. In November, the European Commission unveiled a new stock-take of progress in its Bioeconomy Strategy, ahead of an official revision planned in 2018. In a manifesto, stakeholders demand a holistic policy framework.
Antimicrobial resistance:?In 2017, the development of new drugs and diagnostics to combat multidrug-resistant pathogens gained momentum in Europe. Small and medium-sized companies (SMEs) successfully raised financings and grants – and some new start-ups entered the scene. To continue this trend, specific support for SMEs is needed, says the BEAM Alliance.
As Big Data analytics begin to inspire medical decisionmaking, companies and scientists have gone to war over access to data derived from patient biopsies. Cancer profiling is a seminal situation. While some companies are seeking to profit from approaches to advanced mutation analysis, researchers are vociferous in demanding open access to proprietary databases behind company firewalls.
Pharmapack 2018 Focus:?The dawning age of digital healthcare is expected to transform the pharma packaging and drug delivery sector. At the 21st edition of Pharmapack Europe (7-8 February, 2018, Paris), an expected 5000+ professionals and 400+ exhibiting companies – the industry’s largest meeting ever – will discuss the latest innovations in serialisation, connected devices, and digitalised meds.
It’s still under the radar of analysts. However, the vasoactive peptide adrenomedullin could become the Next Big Thing in therapy monitoring and the treatment of disorders caused by endothelial dysfunction such as acute heart failure, or septic shock. During a scientific symposium Endothelial Dysfunction – Adrenomedullin as a diagnostic and therapeutic target (19. December 2017, Berlin), 15 international medical key opinion leaders from ICU/ED’s for the first time compiled clinical data from more than 10,000 patients that underscore that adrenomedullin could lead to a paradigm shift in the diagnosis, monitoring and treatment of disorders linked to endothelial dysfunction.


![EB_Winter_2017_nasamu_Malaria-in-cell_tg.jpg An electron micrograph of a malaria parasite inside its host red blood cell, ready to burst out. The small merozoites will each find a new red blood cell to invade and grow within. (Picture: Armiyaw Nasamu and Wandy Beatty, Washington University])](https://european-biotechnology.com/wp-content/uploads/2024/04/EB_Winter_2017_nasamu_Malaria-in-cell_tg-180x180.jpg)