Outgoing EU Commissioner for Agriculture Paul Hogan said in April, that the European Commission will exclude member states from the future Common Agricultural Policy (CAP) that do not include the promotion of the bioeconomy in agriculture.
ADVERTISEMENT
The European Council, the European Parliament, and the outgoing European Commission have agreed upon the priorities and structure of Horizon Europe (2021 to 2027), the next seven-year research and innovation programme of the European Union. This autumn, they will decide on the Parliament’s proposal to increase its budget from €100bn to €120bn.
After the birth of the first genome-edited babies in China, regulators, researchers, and bioethics experts seek ways to ban clinical use of germline editing, in order to protect the innovative field from misuse for human enhancement and side effect-prone application forgene correction and human enhancement.
There is a growing demand for more patient-centric drug delivery and improved health-care cost management via self-administration and smart devices. The global drug device combination market is projected to reach nearly US$140m by 2025. It’s where the two different worlds of pharmaceutical (GMP) and standardised medical devices collide.Each needs to learn from the other in order to facilitate the regulation process in Europe.
Targeted therapies and chemotherapeutics have helped cut cancer deaths dramatically in the last few decades. In the US, the overall mortality rate of 251/100,000 patients in 1991 had fallen by 2016 to 156/100,000 patients. Engineered (multispecific) antibodies, cell therapies and combination therapies are expected to continue to increase success rates with primary tumors. And research groups are turning their big guns on what is now the biggest killer – metastasis.
Pilot routine tests with the vasoactive biomarker bio-ADM confirm clinical test results. Medical leaders back actionable utility of the marker in medical decision-making in septic shock and congestive heart failure.
Biomanufacturing – either for production of biologics or bio-based everyday products – has traditionally suffered from the unpredictably variable response of production cell lines to external stimuli. Synthetic biology groups now wish to create a network of biofoundries that aim to establish reproducibility standards from process design to product purification and – in the long term – intend to switch from cell-based to cell-free bioproduction.
As resource-poor biotech start-ups are the drivers of innovation in the biotech sector, partnering with Big Pharma is essential to survive in a competitive environment. Yet, how to do most effectively? The most often held pharma partnering conference worldwide, euroPLX, provides a plausible answer.
The EU’s Big Pharma companies have sharply criticised the European Parliament’s Legal Committee JURI backing of an amended proposal of the European Commission (EC) to introduce a supplementary protection certificates (SPC) manufacturing waiver. Under the waiver, EU-based biosimilar and generics makers would be allowed to export copycat drugs during the SPC period that adds five years of additional IP protection to the 20 years of patent duration.
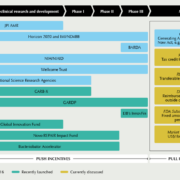
Financing of AMR innovation is shifting gears in early 2019. US AMR accelerator CARB-X has expanded its global reach, the Novo REPAIR impact fund reflects on their first and upcoming investments, and the British Government is underway to take first steps for a pull mechanism this year. During the Novel Antimicrobials and AMR Diagnostics conference in mid-March in Berlin, 300 international AMR experts will discuss the status quo.


![eb_2019_Directorate-General_for_Agriculture_and_Rural_Development_in_Brussels.jpg Directorate-General for Agriculture and Rural Development in Brussels. Picture: Hanna Penzer [CC BY-SA 4.0]](https://european-biotechnology.com/wp-content/uploads/2024/04/eb_2019_Directorate-General_for_Agriculture_and_Rural_Development_in_Brussels-180x180.jpg)

 123rf.com/valentinakru
123rf.com/valentinakru