Dreams of simple, reliable and scalable processes for the much-hyped cell therapy market have generated demand for cost-effective, GMP-compliant manufacturing methods. The drug-screening market is also hungry for material that will allow the establishment of human cell disease models. A growing number of companies are now trying to coax induced pluripotent stem cells (iPSCs) into specific cells needed for clinical trials. Others are seeking the same goal through direct transdifferentiation protocols. What method will prove superior?
ADVERTISEMENT
Despite the threat of a looming climate catastrophe, low oil prices and giant industrial conglomerates addicted to fossil resources continue to stifle industrial biotechnology products and innovation. The European Biotechnology Network is now providing a foot up for companies that want to help themselves by launching three very different projects aimed at pushing the bioeconomy forward.
Enzymes have been used widely for years to bleach textiles or shrink wool. However, the clothing sector is one of the world’s most polluting industries. And now fashion labels are turning increasingly to sustainable biofabrication and biotech-inspired methods to help lower the environmental footprint of their products.
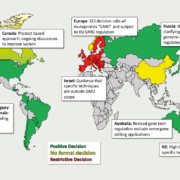
After years of regulatory deadlock concerning political decision making on genome-edited and genetically targeted mutational breeding, the European Council has demanded that the European Commission regulate new plant breeding techniques (NBT), such as oligonucleotid-directed mutagenesis (ODM), gene scissors (CRISPR), and others.
Despite challenging markets new players are entering the field to advance drug development in the fight against antimicrobial resistance.
SMEs active in antibiotic development are trying to adapt to the uncertain AMR business. Meanwhile, the European IMI AMR accelerator kicked off.
Antibiotic developers struggle with uncertain market conditions, weak reimbursement schemes and low turnover once a drug is approved. Experts are trying to figure out the best way to balance push and pull incentives.
Although it has only been shown to provide partial protection, the first-ever malaria vaccine is in testing in Africa – and there are many more hopefuls in the pipeline. Will we soon eradicate one of the most deadly pathogens in human history?
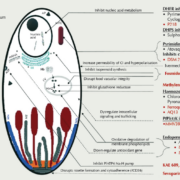
Tackling the malaria pathogen P. falciparum has been complex, due to rapid development of resistance. A new compound targets liver stages of the parasite and, thus, provides a new mode of action to reduce the population of the parasite.
It’s no easy task to develop medications that are effective and safe to use. The pharmaceutical industry loses billions every year due to safety-related attrition during the drug discovery process. For both patients and companies, improving productivity in the area would bring significant benefits. Now novel biomarkers designed to better detect and manage drug-induced organ injury – both preclinically and clinically – could make the process faster, safer and cheaper. tg


![2019_11_05_staphyllo_sw.jpg Source: Eric Erbe, Christopher Pooley [Public domain]](https://european-biotechnology.com/wp-content/uploads/2024/04/2019_11_05_staphyllo_sw-180x180.jpg)