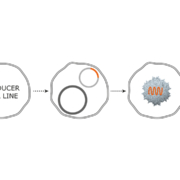
Biotechnology is a key market segment for Coherent. We provide innovative solutions for academic research, pharma discovery, translational research, clinical testing, and interventional therapeutics. We support these applications with lasers, laser engines, fibers, and fiber optic components.
ADVERTISEMENT
In the 1990s, the first clinical IVR systems (Interactive Voice Response Systems) were developed in order to randomise patients over the phone, and later, to dispense drug and resupply sites as well.
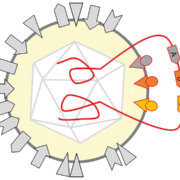
With the rapid developments in the field of gene therapy, the use of viral vectors, especially AAV, to treat various monogenic, inherited diseases in human patients is gaining momentum. Nonetheless, there are still options to optimise the production of such vectors in mammalian cells.
This year, Merck KGaA Darmstadt, Germany has acquired AmpTec, an internationally active
contract manufacturing company (CMO) with comprehensive services for manufacturing of synthetic RNAs and mRNAs and more than 15 years of experience, with cGMP compliant manufacturing capabilities in development. This marks an exciting new chapter, where our combined expertise will allow us to offer our customers innovative technologies and products to help advance life-enhancing therapeutics.
In clinical trials of new drugs and vaccines, the analysis of peripheral blood mononuclear cells (PBMCs) is increasingly recognized as a valuable tool to get insight into the cellular immune response. These data can be used as surrogates in clinical trials for evaluation and market authorisation of new prophylactic or therapeutic vaccines. The collection of the drawn blood and its transport to an isolation lab is a logistical challenge that requires sophisticated procedures and diligent oversight.
Since virus immunotherapies have the potential to improve therapy responses, we established a platform technology based on Herpes Simplex Virus Type 1 (HSV1). TheraVision constitutes a complete development pipeline including a proprietary HSV1 engineering vector for the modular integration of transgenes, a GMP-compliant production process, and complex preclinical test systems including human tumour and immune cells to demonstrate sensitivity and safety.
Cancer immunotherapy exploits the body’s own immune system to fight against cancer. CAR-T cell therapy is one of the breakthrough approaches that involves re-engineering a patient’s own
T cells to recognize and eradicate cancer.
Early December, Deutsche Börse has started BIOTECH Insight, a new information service on the German biotech industry.
Building on a relentless commitment to customer service and innovation, Beckman Coulter Life Sciences debuts a powerful new interactive customer resource with the launch of its Digital Campus. The 3D platform provides greater access to the company’s resources regardless of physical location and allows users to control their experience including the chance to get up close and personal with products and experts.
On 7th July 2021, the German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte – BfArM) launched the first sale of cannabis exclusively for medical purposes from cultivation in Germany. Pharmacies are now able to purchase medical cannabis flowers of pharmaceutical drug quality for the supply of patients from the BfArM via the official portal of the German Cannabis Agency (www.cannabisagentur.de).