Whether it´s oncolytic viruses or vectors for cancer therapy or modern vaccines, the demand for development, production or filling capacities is rising rapidly. To keep pace with this rise in customer demand, Vibalogics, which recently became part of the Recipharm Group, is investing around US$50m in the expansion of its Cuxhaven site by the end of the year, and even more in the US site near Boston.
ADVERTISEMENT
In the present newsletter special issues will be reinforced and personality of branches interviewed. Financing in sustainable production (#BRAIN_AG), synBio and AI (AIgnostics) are highlights in the Biotech Newsletter of Deutsche Börse AG.
Immunogenicity and limited gene transfer capacity can negatively affect the outcome of cell and gene therapies. European Biotechnology magazine spoke to Dr Dimitrios Laurin Wagner, Berlin Center for Advanced Therapies (BeCAT) and Gene Editing for Cell Therapy group leader at Charité, on new approaches that promise to overcome some limitations of current virus-based gene therapies.
The technological and pharmacological advances lead to an increase in the number of molecules for R&D that are challenging and difficult to manufacture. To improve clinical success, pharma and biotech companies are seeking innovative ways to accelerate progress and reduce scientific, economic, and delivery risks.
A current Nature study specifies the most competitive health biotech sectors worldwide and puts Switzerland at the top, Sweden second and the USA third. Does the new math convince?
Vetter, a globally operating CDMO, won the 2022 CMO Leadership Awards in all six core categories – quality, expertise, compatibility, capabilities, reliability and service. Vetter also achieved Champion status in the areas of quality, expertise and compatibility. The award, now in its eleventh year, was given by the leading trade press publication, Life Science Leader.
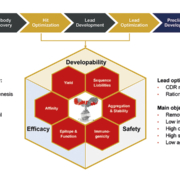
The efficacy of therapeutic antibodies is defined by their primary amino acid sequence. After discovery, antibody candidates often profit from protein engineering and sequence optimisation. Different technologies can improve the functional properties of antibodies as well as their biochemical and biophysical characteristics influencing manufacturing, process development, formulation, and other parameters of the final drug target product profile.
Biologicals have shown significant success for more complex diseases, whereas small molecule drugs have not been that effective. New biologicals are mostly highly purified proteins or antibodies, which require the development of technologies for the preparation of a large number of drug candidates with high purity and speed. A high throughput magnetic beads-based system is a game-changer for biologics discovery.
In recent years, several beneficial cell samples and microbial strains have been discovered, engineered, and studied, resulting in scientific discoveries and other technological breakthroughs such as vaccines and medicines benefitting communities all around the world. However, several medical products tend to require very low temperatures for storage, making Ultra-Low Freezers increasingly important to maintain their viability.
Data Management has been a hotbed for development and innovation, correlating primarily with
the changing digital landscape. Clinical trial management has been significantly impacted, with the
electronic data capture and GDPR-compliance-led industry now unrecognisable from the paper-document administration methods of the past. This development process extends beyond COVID-19 and has a
noticeable impact on clinical trials.