Viral Vectors?Commercial-scale manufacturing demands a high yield of potent rAAV products. Understanding the metabolism during cell growth, especially at high densities, molecular effects of transfection or infection in production is vital. In addition, the choice of producer cell type determines the PTMs of the AAV capsid and potency. Successful trials in tweaking cell factories’ genes to boost rAAV particle yields set the direction for further development.
ADVERTISEMENT
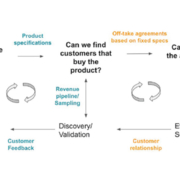
Precision gene delivery remains one of the greatest challenges in modern medicine. A Basel-based biopharma company, Vector BioPharma, has accepted the challenge and, with cutting-edge technology from the University of Zürich, is developing a revolutionary new way to deliver the right genes to the right place at the right time. Vector BioPharma aims to be the pioneer in delivering future medicines with this technology, now under development in multiple areas of urgent medical need.
As a late-stage investor focusing on the European bioeconomy, the ECBF is carefully assessing the maturity of any technology, but more important than maturity is the ability to reach commercial scale. What governs that scale-up, and how do we assess it? What role does the TRL score play, and what key questions do we ask? An excerpt into a framework.
CB21 Pharma Ltd announces that it entered a global strategic collaboration to combine
its leading expertise and clinical research program in phytocannabinoids with world-class
pharmaceutical strategic consulting and business development services of NorthStar Corporate Finance Ltd and Kru?ger Beteiligungs GmbH, forming a new company called CB21 R&D.
BioFIT is Europe’s leading partnering event in Life Sciences for tech transfer, early-stage innovation deals and pre-seed, seed and Series A investment, receiving each year more than 1,000 delegates from over 35 countries. For its 11th edition, BioFIT will be held in Strasbourg, France on November 29th and 30th, and on a digital format on December 7th and 8th.
Takeda, Boehringer Ingelheim and Novartis do it repeatedly. Global players make major investments in their Austrian facilities. Not only multinationals but start-ups and scale-ups such as Apeiron Biologics and ViraTherapeutics benefit from a comprehensive ecosystem – from basic research to production and distribution. The business location agency ABA is the first point of contact for setting up operations in Austria.
Keeping biologicals at low temperatures is an established and effective way of maintaining their potency. This is critical for many types of medicines, vaccines, reagents, and biological samples, which is the reason why laboratories, hospitals, clinics, pharmacies, and more employ medical-grade refrigerators to protect their samples from ambient conditions.
After two years online, PharmaLab 2022 returns on site for its 10th edition. Over three days, the congress will offer the opportunity to share the latest experiences with colleagues and to exchange ideas in person.
The CRO industry is predicted to grow considerably over the next five years. Trials are becoming more complex, and technology is becoming increasingly interwoven with processes. The CRO of tomorrow must be tech smart, agile, and environmentally conscious to remain relevant. In this changing landscape, how does a CRO cut through the noise in 2022-30?
To install a newly created Chief Scientific Officer position and to hire a biotech veteran is a signal sent by Rentschler Biopharma, celebrating 150 years.





 ABA Austria
ABA Austria

