Financing AMR innovation discussed globally
Financing of AMR innovation is shifting gears in early 2019. US AMR accelerator CARB-X has expanded its global reach, the Novo REPAIR impact fund reflects on their first and upcoming investments, and the British Government is underway to take first steps for a pull mechanism this year. During the Novel Antimicrobials and AMR Diagnostics conference in mid-March in Berlin, 300 international AMR experts will discuss the status quo.
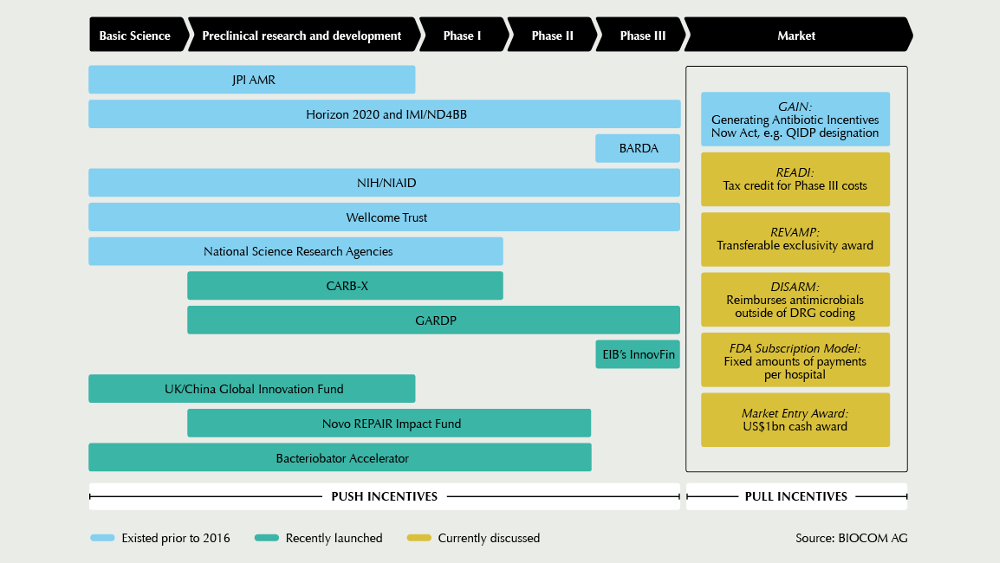
The new year has kicked-off with promising news: In February, one of the main financiers in the field, US AMR accelerator CARB-X, announced its plan to expand its reach by adding six partners from Germany, Switzerland, Denmark, and India to its network. Launched in the US by BARDA in 2016, CARB-X has a budget of US$540m from 2017 till 2021 for antibacterial R&D, particularly for investments into the development of new drugs against high-priority, drug-resistant Gram-negative bugs. The R&D network currently funds 34 projects, including new antibiotics, rapid diagnostics, vaccines, and other products that target drug-resistant bacteria. In the long-term, CARB-X aims to increase its portfolio to up to 60 projects, and for this, accelerator expansion is needed, says CARB-X’s Executive Director Kevin Outterson.
CARB-X partners with DZIF
Among the new partners is the German Centre for Infection Research (DZIF). If we do not want to be left without effective antibiotics in ten years’ time, we need to establish new, strong consortia now, Thomas Hesterkamp, responsible for product development, explains. His unit will not only accompany project teams in their CARB-X funding process, but also provide advice for project implementation. Located in Copenhagen, Denmark, the BioInnovation Institute, an initiative of the Novo Nordisk Foundation, was chosen as another new CARB-X partner. The start-up hub offers lab facilities, as well as business acceleration support. I expect to see many, new anti-infective programs targeting AMR accelerate in BII and graduate to further funding from the REPAIR Impact Fund, commented Aleks Engel, Director of the REPAIR Impact Fund, which was set up one year ago as a new €135m investment vehicle also by the Novo Nordisk Foundation. By the end of 2018, the first €18m have been injected into Polyphor (CH), Procarta Biosystems (UK), Entasis Therapeutics (US), and Minervax (DK). The fund was launched to help fill a critical gap in early-stage equity funding for anti-infective therapies, specifically between the beginning of lead optimization and the end of Phase I, Engel says. According to him, the first lesson learned from this initial year is how financially devastated the market for anti-infectives has become – both from a revenue-generating context and from an investment perspective. Since the launch of the fund, the share prices of the 13 existing, publicly-traded, anti-infective-focused biotech companies have fallen by more than 60%, and Allergan, Novartis, and Sanofi have left the field, he states. For this reason, his outlook is mixed: I am a firm believer that good things will happen when the cutting-edge, early-stage pipeline today hits the unmet needs of the market in four to seven years. Unfortunately, I am doubtful about the commercial prospects of the less differentiated, late-stage pipeline right now, and thus, the market decline may continue for another few years.
In view of this situation, initiatives such as those in United Kingdom to start implementing pull incentives have been welcomed by the AMR community. It is very good to see action after many years of reports, and we thank the UK for making the first move, says Marc Gitzinger, CEO of Swiss Bioversys and Vice-President of the SMEs organisation BEAM Alliance. Another step forward could be a new, global AMR health care impact investment fund targeting the financing of late-stage clinical projects (Phase II and III). Currently, we are trying to determine the feasibility and possible structure of such a fund, in particular, the key economic figures and the actual necessity for specific de-risking strategies to guarantee a minimum return, explains Peter Beyer from the World Health Organization. In a workshop at the Novel Antimicrobials and AMR Diagnostics conference in Berlin, at which more than 300 experts are expected, stakeholders are invited to comment on the first conceptional ideas. Based on the feedback, WHO will work with the World Bank, other development banks and financial consultants to refine the concept.
Leverage Next-Gen-Sequencing for AMR research community
Meanwhile, the industry is continuing its search for new tools to improve the fight against superbugs. For instance, in February, Austrian Ares Genetics joined forced with German diagnostics company Qiagen to leverage Ares Genetics’ antimicrobial resistance database, ARESdb, as well as bioinformatics tools and workflows as a new service for the research community. The database offered by Ares, a subsidiary of listed Curetis, contains over 10,000 whole genome-sequenced clinical isolates collected from more than 200 centres globally. It combines broad resistance profiles with high-resolution, genetic information and enables accurate pathogen identification and already allows very precise, quantitative antibiotic resistance prediction for more than 150 drug/pathogen combinations, says Achim Plum, Managing Director of Ares Genetics. He adds: With the collaboration of Qiagen, we paved the way for external validation of this new resource within the AMR research community. In the mid-term, the company will collaborate with industry partners and seek to leverage ARESdb to develop novel AI-powered diagnostic solutions and to support the discovery and clinical development of antibiotics. In December, pharma company Sandoz started working with Ares Genetics on a cost-effective AI-approach to re-purpose existing antibiotics and expand their indications.
First published in European Biotechology magazine, Spring 2019 Edition.


 Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany
Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany Sitryx Therapeutics
Sitryx Therapeutics