
Ensuring flexibility and resilience
In today’s rapidly evolving world, adapting to demand volatilities while ensuring uninterrupted supply of biological medicines is more critical than ever. At Boehringer Ingelheim, our global biopharmaceutical network delivers flexibility, resilience, and reliability. By leveraging harmonised procedures, standardised setups, and innovative logistics, we ensure the biologics we produce reach those in need – even during unexpected challenges.
Boehringer Ingelheim BioXcellence™ operates large-scale cell culture facilities strategically located in the United States and Europe (Austria and Germany). This global footprint provides robust and reliable solutions to meet the evolving needs of the biopharmaceutical industry.
Strength of our global network
Our European facilities in Biberach, Germany and Vienna, Austria feature similar technical setups, ensuring positive technical comparability and thus consistent product quality. This harmonisation is key to maintaining the highest standards across our network. Even slight differences between our U.S. facility in Fremont, California, and the European sites – such as stirrer or sparger configurations – are seamlessly managed thanks to our experience and operational excellence. This expertise in the network allows us to predict and address potential variations, ensuring smooth process transfers both for client-specific processes into our network and in case an additional supply node is requested by our customers also within our network.
One key idea is to develop robust and easily transferable production processes for large-scale manufacturing. This approach might offer the option to skip engineering runs, enabling us to immediately proceed with process performance qualification (PPQ) runs after completing small-scale runs, in vitro comparability studies, gap and risk assessments, as well as simulations and modeling.
By streamlining the manufacturing process, this method could save valuable time and resources. Not only does this approach enhance flexibility in supply for our customers, but it also accelerates the delivery of critical medicines to patients in need. This idea of ‘portable’ production processes exemplifies our commitment to innovation and efficiency.
Standardisation drives flexibility
Whether responding to demand fluctuations or timeline variability, our global manufacturing network setup enables interchangeability and a reliable supply without compromising quality. This consistency is achieved by a high level of process standardisation, thorough planning and execution, ensuring that every product meets the highest standards, regardless of the manufacturing site.
To further strengthen operational resilience for our customers, we offer access to our cryovessel pool and our newly established Global Vessel Management Center – developed in collaboration with a trusted logistics partner to deliver seamless, reliable, and expert solutions. Designed for technical excellence, the Global Vessel Management Center offers validated cleaning processes, secure storage conditions, and comprehensive maintenance programs to support operational efficiency and regulatory adherence.
Resilience amid uncertainty
The COVID-19 pandemic and ongoing geopolitical uncertainties have underscored the importance of robust supply chains. That is why we have implemented a comprehensive Business Continuity Management (BCM) system designed to anticipate and mitigate risks, ensuring that our operations remain reliable under any circumstances.
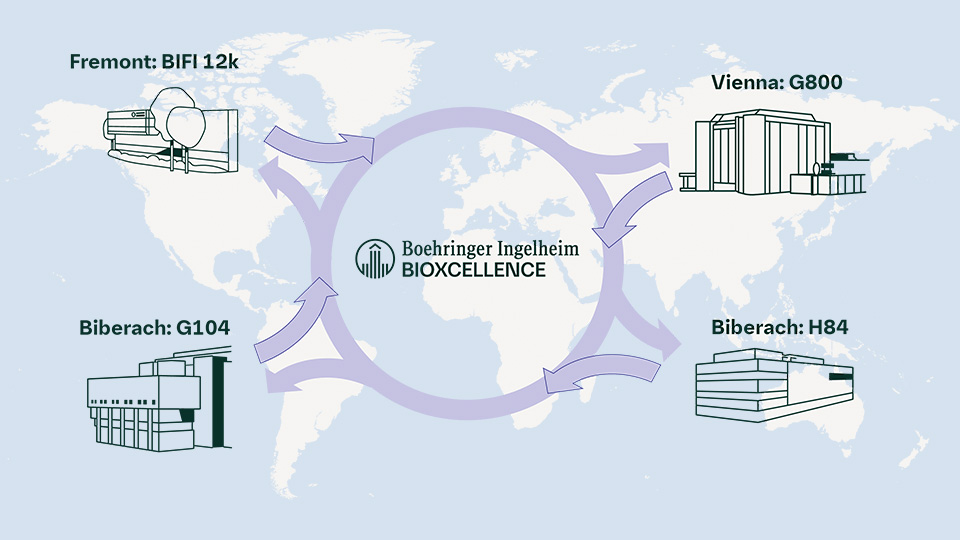
Figure 2: The standardised setup of bioreactors and harmonised procedures across our large-scale cell culture facilities offer interchangeability, providing high flexibility and reliable supply security for our partners.
© Boehringer Ingelheim Biopharmaceuticals GmbH
Key pillars of our BCM framework
Our business continuity management framework is built on three dimensions.
1. Enhancing production continuity for patient supplies: Our commitment to business continuity ensures uninterrupted operations, allowing us to reliably meet our customer’s needs and deliver on time, even in the face of unexpected challenges.
2. Managing contractual obligations: Customer requirements for BCM systems are integrated into our contracts, and regular audits ensure the effectiveness of our measures. This proactive approach builds trust and accountability with our partners.
3. Maintaining customer trust: Reliable delivery is essential to preserving our reputation and relationships with clients. Our BCM system mitigates the risk of disruptions that could impact trust, ensuring that our partners can rely on us.
Advantages for our customers
Our global biopharmaceutical manufacturing network offers several key benefits that make Boehringer Ingelheim
BioXcellence™ the partner of choice for large-scale cell culture contract manufacturing.
First, our network ensures resilient supply chains, even in times of geopolitical uncertainty. By leveraging our distributed facilities, we minimise risks and maintain a reliable delivery of critical medicines. This capability is particularly valuable for customers seeking stability and consistency in their supply strategies.
Second, we offer enhanced flexibility in responding to demand volatilities. With multiple sites registered for production, we can shift manufacturing runs between facilities to align with clients’ supply strategies. This adaptability allows us to meet changing market demands while maintaining high standards of quality and efficiency.
Third, our streamlined oversight processes reduce the complexity for clients. Customers with multiple sourcing supply security strategies can combine in-house production capabilities with various sites at one contract manufacturing organisation (CMO). This approach simplifies oversight efforts, allowing customers to focus on their core operations while trusting us to manage the complexities of manufacturing and logistics.
Finally, our harmonised setups and standardised processes ensure consistent product quality across all sites. Customers can trust that their products will meet the highest standards, regardless of where they are manufactured within our network.
Many of our customers have established their products at more than one site within our network, enabling us to discuss and adopt production schedules and jointly optimise supply strategies. This flexibility is a critical advantage in today’s dynamic market environment. By working closely with our partners, it is our ambition that their needs are met with precision and care, fostering long-term relationships built on trust and reliability.
At Boehringer Ingelheim BioXcellence™, we’re committed to leveraging our global biopharmaceutical manufacturing network to its fullest potential. By combining harmonised procedures, logistics systems, and business continuity management, we deliver for our partners and patients.
As the world continues to face challenges, our large-scale mammalian cell culture production network stands as a testament to the power of collaboration, innovation, and operational excellence
This article from Dr Rebekka Wuester, Communication Lead BioXcellence, Biopharmaceutical Contract
Manufacturing Business, Boehringer Ingelheim Biopharmaceuticals GmbH, was published in European Biotechnology Magazine Summer 2025.


 Boehringer Ingelheim
Boehringer Ingelheim Boehringer Ingelheim
Boehringer Ingelheim