Researchers find clue to Parkinson’s gene therapy
An enzyme that clears out alpha-synuclein from neurons may counteract Parkinson's Disease, report researchers from ETH Zürich and Novartis.
The scientists headed by Juan Gerez and Paola Picotti identified a biochemical process in mice models that breaks down the accumulation of proteins associated with Parkinson’s disease (PD). Though further preclinical testing is needed, the results suggest that enhancing this natural process could form the basis of new treatments for PD and related neurodegenerative disorders.
Protein aggregates called Lewy bodies drive the progression of Parkinon’s disease in the brains of patients. Lewy bodies are composed of ?lpha-synuclein, which spreads in the nervous system through secretion and uptake by neurons. So far, scientists did not understand how the protein accumulates within neurons.
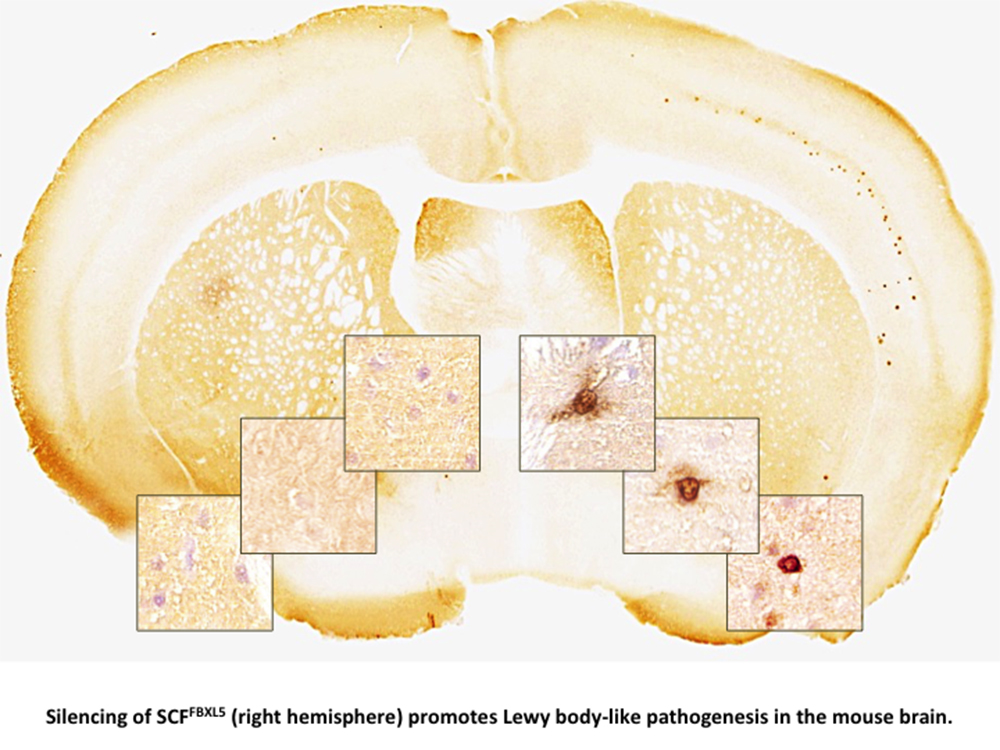
Seeking additional insight, Aguzzi and colleagues treated human neuroblastoma cells and other cell lines with different molecular forms of ?lpha-synuclein, and saw that the accumulation of ?lpha-synuclein fibrils – rod-like protein structures – within the cells triggered a response. Specifically, the neurons released an enzyme complex named SCF that broke down ?lpha-synuclein fibrils through ubiquitination. SCF also blocked the propagation of ?lpha-synuclein between cells but did not prevent the transfer of ?lpha-synuclein to cells lacking Cul1, a component of SCF.
Promoting ubiquitination with a virus-based delivery system prevented the formation and spread of ?lpha-synuclein and Lewy bodies in mouse models of ?lpha-synuclein accumulation. Several drug discovery programmes have been launched to investigate the potential of modulating SCF to treat PD in humans.



 Microbiotica
Microbiotica Araris Biotech AG
Araris Biotech AG