Liquid biopsy identifies residual breast cancer
Using a platform tailored to patients' specific cancer mutations, British and US researchers for the first time detected residual disease in breast cancer patients.
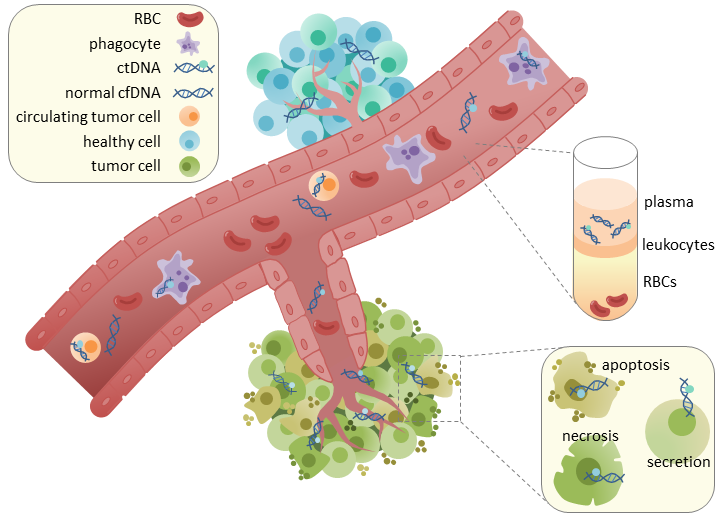
Their new mutation detection platform TARDIS showed a 100-fold improvement in sensitivity compared to established liquid biopsy technologies that identify circulating tumour DNA (ctDNA) in blood. As their study was small, the researchers said more testing is needed to validate the diagnostic potential. However, they believe the assay holds the potential to be used to help monitor how patients respond to chemotherapy like current mRNA profiling tests do and – more relevant – to for the first time identify recurrences of tumours earlier on.
As only very low amounts of ctDNA is found in the blood at early cancer stages or after chemotherapy, compared to the amount of the bulk of circulating DNA, so far is was not possible to detect ctDNA in patients at early breast cancer stages or who had undergone chemotherapy. This obstacle has impeded the creation of a platform sensitive enough to detect signs of recurring disease – a great concern in many cancer patients.
TARDIS is a sequencing platform that analyzes patient-specific mutations from limited sample sizes. The assay analyzes an amount of DNA equivalent to a single tube of blood and can simultaneously study a panel of 8 to 16 known mutations. TARDIS successfully detected ctDNA in plasma samples from 33 patients with breast cancer before they started treatment, and revealed the patients had lower concentrations of ctDNA after treatment was completed. However, the effect size to not to large: patients who responded the best to chemotherapy displayed a 96% decrease in ctDNA abundance, while patients with residual disease showed a 77% decrease. However, according to the researchers it was sufficient sensitive to tell if early stage breast cancer patients have responded well to pre-operative drug therapy. Furthermore, the digital sequencing platform is more sensitive than the current method of determining response to drug therapy using imaging.
In contrastt to histologic biopsies, liquid biopsies use a simple blood draw, and so could safely be performed repeatedly, in order to monitor a patient’s disease status. Next steps also include scaling up the platform for use in hundreds of patients.



 Microbiotica
Microbiotica Araris Biotech AG
Araris Biotech AG