4SC expands domatinostat combo testing
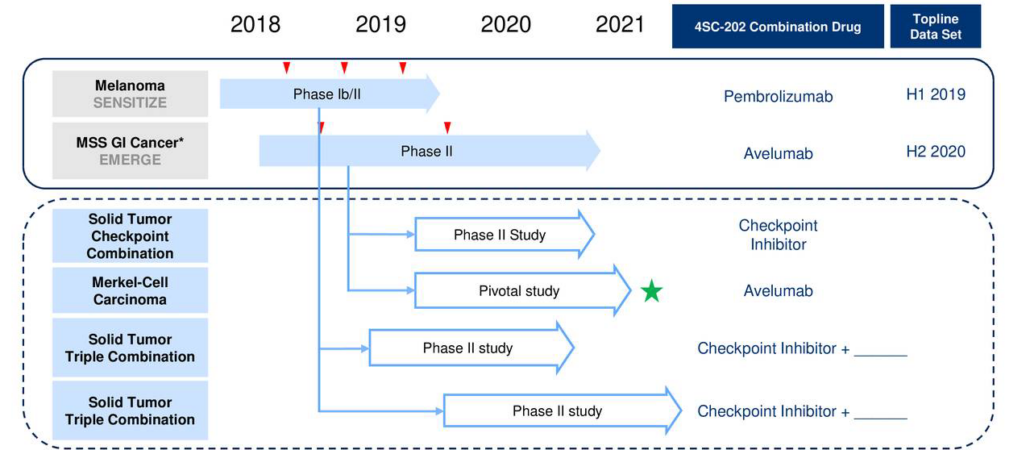
The Netherlands Cancer Institute (NKI) has joined 4SC AG as a partner in conducting a multicentre study of dominatostat/checkpoint blocker combos in treatment-naive melanoma patients.
In an investigator-initiated Phase Ib study (DONIMI) at NKI, 40 patients with melanoma will be enrolled before surgery (neoadjuvant setting) to evaluate 4SC AG’s (Martinsried, Germany) oral class I histone deacetylase inhibitor domatinostat in combination with Bristol-Myers Squibb‘s PD1-blocker Nivolumab and BMS’ CTLA4 inhibitor ipilimumab. Preclinically, domatinostat has been demonstrated to strengthen the body’s anti-tumour immune response by boosting the expression of tumour neo-antigens and immunomodulating molecules in tumour cells and by promoting T cell invasion into the tumour. The small molecule compound also reduced the number of tumour-promoting myeloid-derived suppressor cells in the tumour microenvironment.
Immunotherapy, and particularly checkpoint inhibitors are increasingly investigated not only in the advanced unresectable or metastatic setting but also in earlier stages of disease. The rationale for such treatment is to activate the immune system before resection of the tumour to generate an immune memory and prevent recurrence of the disease. On the basis of some encouraging data published by the International Neoadjuvant Melanoma Consortium (INMC; https://melanoma-inc.org/), combination approaches will now be tested to optimise the treatment in a more personalised manner where domatinostat is a promising combination partner.
It is getting clearer and clearer that immunotherapy for cancer is most effective when used early in disease progression, said Jason Loveridge, CEO of 4SC. We believe the neoadjuvant setting offers a great potential for protecting patients from recurrence of their malignancy after resection, potentially leading to a much higher number of cured patients with resectable, high-risk melanoma.
Principal Investigator Prof. Dr. Christian Blank, NKI, said: Additional combination approaches to modulate the tumour microenvironment and the patients’ immune system remains a priority so as to optimise and personalise treatment for patients with melanoma, and potentially impact the treatment of patients with other cancers. For example, we do not know yet which patients require double neoadjuvant checkpoint inhibition and who can be treated with neoadjuvant PD-1 blockade alone. In the DONIMI study, Domatinostat will be one of the first compounds tested in a personalised neoadjuvant combination trial implemented by INMC researchers from Sydney and Amsterdam. We are much looking forward to enroll the first patients later this year.


 Bayer AG
Bayer AG
 Picture from Ferdinand Stöhr on Unsplash
Picture from Ferdinand Stöhr on Unsplash