
Second clinical failure of Transgene in 2019
French Transgene SA said it has terminated its lead programme TG4010 in combination with chemotherapy and checkpoint inhibition.
French Transgene SA (Strasbourg, France) has announced another study failure, following the Phase III flop of its oncolytic virus programme this summer. We are obviously very disappointed with the outcome of this Phase II trial which showed that the triple combination regimen of TG4010, chemotherapy and nivolumab did not sufficiently increase the response rate in this patient population with advanced Non-Small Cell Lung Cancer (NSCLC) whose tumor express low or undetectable levels of PD-L1," said Philippe Archinard, Chairman and CEO of Transgene. "Additional data analyses are still ongoing and the complete study results will be presented at an upcoming scientific conference.
The Phase II study, which was conducted under a clinical collaboration agreement with Bristol-Myers Squibb, failed to meet its primary endpoint of overall response rate in 40 patients whose tumors express low-to-no PD-L1. The combination regimen was assessed as a first-line treatment for patients with advanced non-squamous non-small cell lung cancer (NSCLC) with low-to-no expression of PD-L1 by the tumour cells (PD-L1<50%). Transgene has stop further development of TG4010.
The company said it is funded through until 2022 and will now focus on its trials with TG4001 in HPV-positive cancers, two trials evaluating TG4050, a trial with TG6002 administered via the intrahepatic artery. According to Archinard, the company plans to submit a clinical trial application in the first half of 2020 for BT-001, the first Invir.IO® oncolytic virus encoding for an anti-CTLA4 antibody. He added. Transgene’s oncolytic virus collaboration with AstraZeneca was making "excellent progress".


 Microbiotica
Microbiotica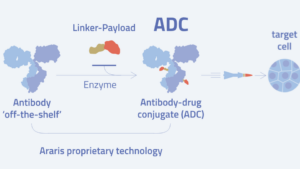 Araris Biotech AG
Araris Biotech AG Freepik.com
Freepik.com