
Manufacturing Biopharmaceuticals
Within the competitive CDMO market, Richter-Helm has developed into a highly valued partner when it comes to the manufacturing of biopharmaceuticals. Richter-Helm is well recognised by pharmaceutical companies, including Big Pharma, as a professional partner offering flexible CDMO solutions from gene to product, all from one source, including consultancy services. This makes Richter-Helm one of the key players in CDMO business.
According to various reports and surveys, the biologics market is constantly growing. Pharmaceutical companies focus on bringing their pipeline products to the market quickly and with minimum risk, and therefore increase their outsourcing activities to reliable partners, such as Richter-Helm. On the other side, CDMOs compete to win the project(s). In the end, decision-making is dependent on the most suitable fit between two partners. The trend seems to go in the direction of one-stop-shop offerings, where projects are outsourced to a single partner able to provide services from process development to analytical development, manufacturing, QC testing and filling of finished product. The goal is to build strong and long-term partnerships on equal footing to strengthen and extend business.
With a 35-year-long track record in GMP manufacturing, Richter-Helm provides its clients with a unique knowledge base in process and analytical validation, process performance qualification (PPQ) procedures, commercial production of therapeutic proteins and peptides, antibody-like scaffolds (e.g., VHH/ Nanobodies, Fab-fragments), bacterial vaccines, and plasmid DNA (pDNA) products.
Service along the value chain
On the company’s sites, new projects can either begin from scratch for full development or involve the direct transfer of existing process technologies into large-scale production sites for market supply or to support with materials for clinical studies and the development of new products.
The increasing demand for further services offers very promising new opportunities for CDMO companies like Richter-Helm with respect to the business growth, the implementation of new technologies, and the establishment of expertise in certain areas. Richter-Helm is using this positive development to expand both its production and development capacities along with its technology pipeline.
To serve the demand for drug production and offer attractive manufacturing solutions for especially large and commercial scales, Richter-Helm is installing a new multipurpose facility, which is designed for maximum flexibility and opens opportunities for new projects. A total area of about 10,000 m2 enables Richter-Helm to take on projects at 300L and 1,500L fermentation volumes for microbial production, as well as related mid- and downstream operations, ensuring high product yields. The new site includes additional space for huge analytical laboratories, warehousing, and technical areas designed for further growth. That makes the delivery of technically advanced services at different scales, especially large clinical scales up to commercial scale, possible.
Via the application and implementation of digital technologies (e.g., digitalisation of process design and targeted process characterisation to facilitate process development) into the whole manufacturing process, the biomanufacturing efficiency at Richter-Helm´s production sites increase further.
This article was originally published in European Biotechnology Magazine Autumn Edition 2023.


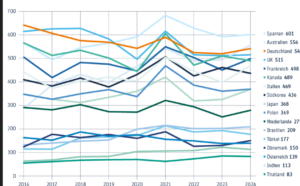 VFA
VFA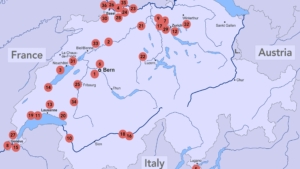 https://www.chimia.ch/chimia/article/view/2025_292
https://www.chimia.ch/chimia/article/view/2025_292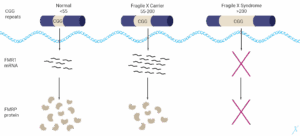 FRAXA Ewsearch Organisation
FRAXA Ewsearch Organisation