Rapid antibody development to cure COVID-19
In addition to vaccination programmes, the SARS-CoV-2 pandemic requires safe and efficient treatment options for patients infected and suffering from Covid-19 disease. Virus-neutralising antibody therapeutics can not only cure COVID-19 but also protect from infection. New fast-track antibody drug programmes have achieved unprecedented timelines from discovery to the clinics and give hope to save lives and cure COVID-19.
Governments have put most of their hopes into vaccines to fight the SARS-CoV-2 pandemic. Whereas Germany invested €750m into three vaccine programmes (BioNTech, CureVac, IDT Biologika), drug development for therapeutics has not been substantially supported so far. In November, BioNTech announced the application for Emergency Use Authorization (EUA) in the US for its novel mRNA vaccine BNT162b2. A good example how financial support spurs innovation and accelerates development. No doubt, an efficient and safe vaccine would be a relief for everybody, but can we sit back and just wait until we get our shot? No! Curative treatment options are still urgently needed because world-wide vaccination will take years. Moreover, there are a number of remaining questions such as the vaccine efficiency particularly in high-risk populations having an increased risk to develop severe disease. In addition, the uptake rates of flu vaccines have been below 40% even in high-risk groups in the past. Last but not least we do not know how long an immune protection will endure. Just for comparison, seasonal flu vaccines achieve protection rates of up to 60%, which would leave many unprotected. Moreover, virus-specific antibody titers of COVID-19 patients vanish rapidly after convalescence, why not also after vaccination?
Accelerating development
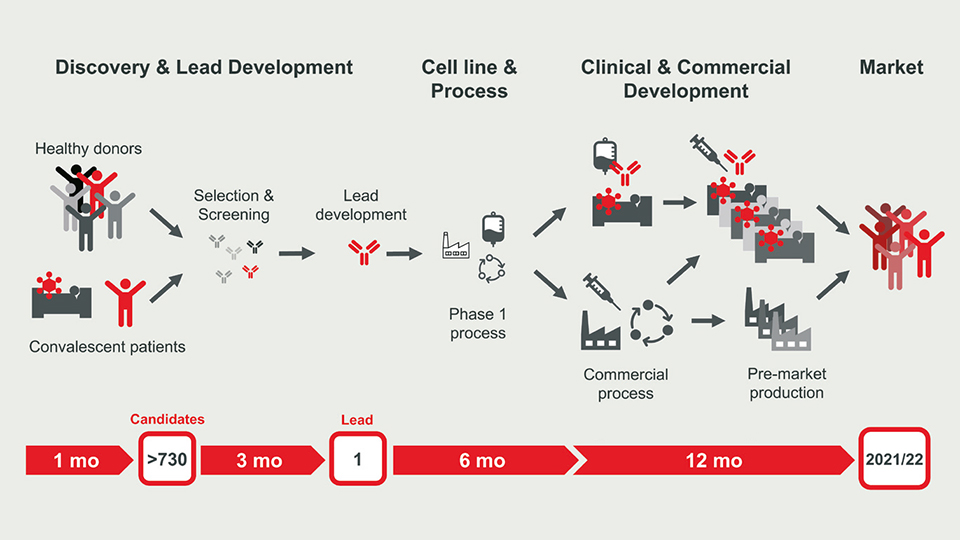
Thus, we need efficient and safe treatment options to cure COVID-19. Great expectations are put upon virus-neutralising antibodies, which provide cure and temporary protection from infection. Integration of fast-track technologies for antibody discovery, cell line development and manufacturing in a parallelised workflow already achieves unprecedented timelines. Corat Therapeutics’ antibody programme required only 4.5 months to develop the lead compound COR-101 and another 3.5 months for cell development and GMP manufacturing. Accelerating clinical testing and ramping up production for the pandemic market under time pressure remains challenging and costs €50m??€100m but it is as critically as any step before.
COR-101 safety design
There are a number of companies developing neutralising antibodies. Regeneron and Eli Lilly are in the lead. In November, Eli Lilly’s antibody LY-CoV555 received an EUA for US high-risk patients with mild to moderate COVID-19. However, clinical data regarding dose and clinical efficacy as well as safety are still not complete and need to be continuously monitored. For example, insufficient antiviral antibody titers can enhance infection and inflammation in COVID-19 a side effect which is well known as antibody dependent enhancement (ADE) and is related to the interaction of antibodies with a certain class of receptors named Fcg-receptors. Corat Therapeutics’ COR-101 employs a novel Fc design to avoid any immunological safety risks of conventional antibodies such as but not limited to ADE. COR-101 combines a superior safety profile with excellent virus-neutralisation, tolerance to common viral mutants and good manufacturability. The product will be tested in patients in early 2021 and is expected to reach the market in early 2022.
BU: Fast track to market accelerated antibody drug development. Picture: Corat Therapeutics GmbH


 Photo from Giulia Bertelli on Unsplash
Photo from Giulia Bertelli on Unsplash  Engitix
Engitix Evotec SE
Evotec SE