SynaptixBio Ltd gets FDA ODD for leukodystrophy
Rare disease specialist SynaptixBio Ltd has got its second FDA orphan drug designation for a subtype of TUBB4A leukodystrophy.
Using antisense oligonucleotides (ASO) to silence the beta tubulin 4a gene, Oxford-based SynaptixBio Ltd received its first ODD in early 2023 for a therapeutic that targets hypomyelination with atrophy of the basal ganglia and cerebellum (H-ABC), the most severe form of TUBB4A leukodystrophy, which represent 9% of all leukodystrophies. A newly ODD granted by the US FDA allows research and development of a therapy for another form of the disease called isolated hypomyelination.
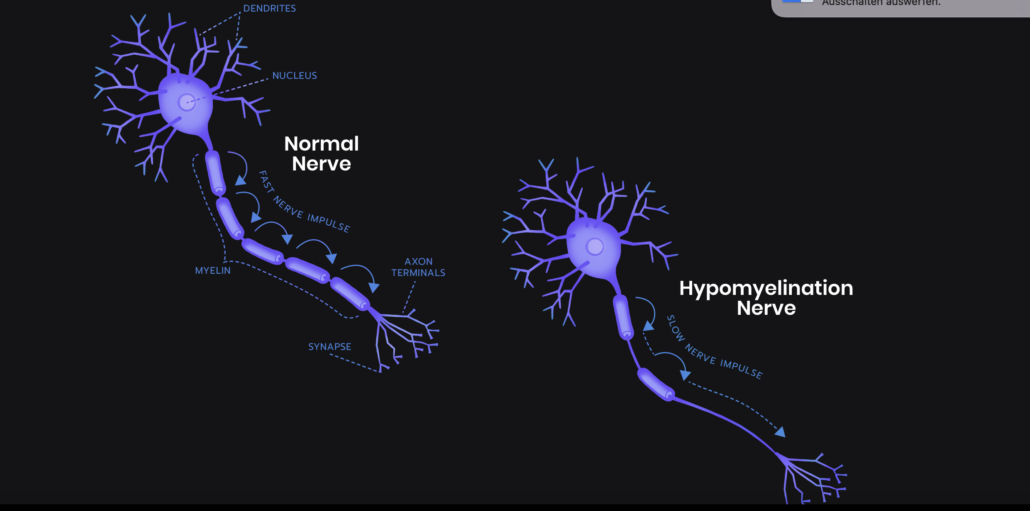
Isolated hypomyelination appears similar to H-ABC but without atrophy of the basal ganglia and cerebellum. The symptoms are reported to be milder than H-ABC. Leukodystrophies affect the brain and manifest with a spectrum of severity and symptoms. Onset is typically recognised in toddlers with developmental delays and deterioration of motor function (gait dysfunction and difficulties with sitting, speech and swallowing). Often survival is limited to the stage of young adulthood.
SynaptixBio is using antisense oligonucleotide (ASO) technology to tackle TUBB4A-related leukodystrophies; ASOs can alter the expression of genes, in this case a specific ASO molecule targets the mutated TUBB4A gene to stop it forming toxic proteins, which in turn help build the cells that form myelin sheaths surrounding nerve fibres in the brain. With the toxic protein suppressed, other proteins step in to help form normal myelin.
SynaptixBio was late in 2023 awarded a £490,000 BioMedical Catalyst grant from Innovate UK specifically to tackle less common variants of the disease, so this ODD award represents the next step in their search for therapies. Earlier last year, SynaptixBio led a second round of investment, taking the total up to £13.2m, which will take it up to the start of in-human clinical trials later this year.
The ODD will enable SynaptixBio to get tax credits, secure grants to offset development expenses, and gain exemption from some pre-marketing authorisation requirements and regulatory fees. In addition, it grants after approval a potential seven years of market exclusivity and data exclusivity.
This second ODD designation follows the award late last year of a Rare Paediatric Disease Designation (RPDD), which can lead to the award of a Priority Review Voucher (PRV) once a product is approved.
A PRV can expedite the FDA’s product review time, and it can be sold or transferred, for example to one of the big pharmaceutical companies (Big Pharma), which can potentially offset the high costs associated with the development of rare disease therapies.




