German pharma association publishes clinical trial ranking
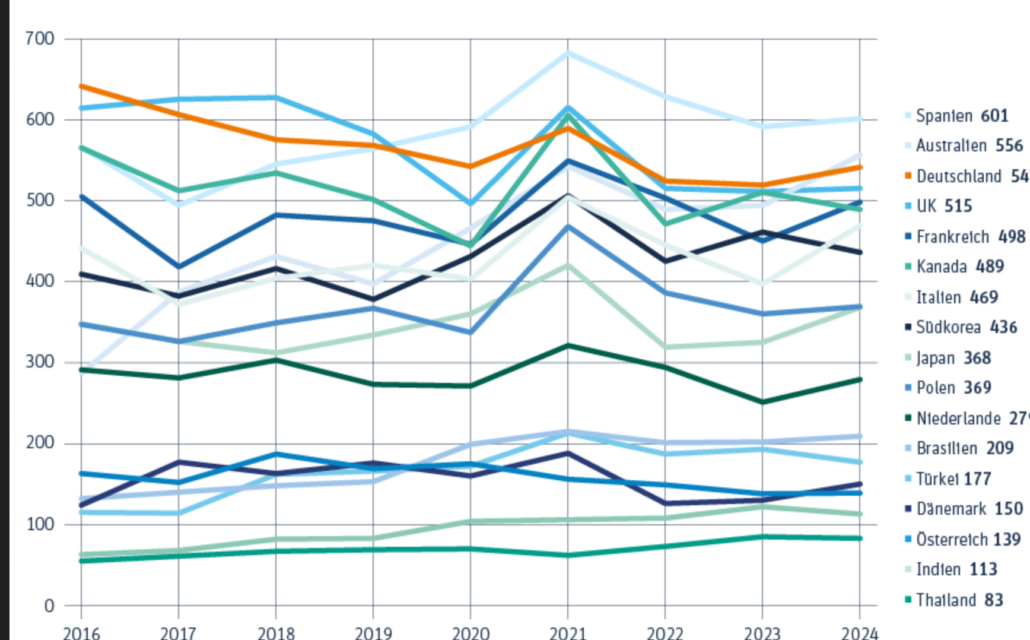
The German pharmaceutical association vfa has updated its ranking of the best locations for industry-sponsored clinical trials. In Europe, Spain retained its top position in 2024, followed by Australia, Germany, the UK, and France.
The German pharmaceutical association vfa has updated its ranking of the top locations for industry-sponsored clinical trials. In 2024, Germany moved up in the rankings, while Spain remained Europe’s leader. Globally, the USA and China continue to lead, with cancer and immunology studies dominating the research landscape.
“Germany has moved slightly upwards, but Spain remains the clear number one in Europe with 601 conducted studies. Australia (556 studies) has overtaken Germany (541),” said vfa spokesperson Dr. Rolf Hoemke to European Biotechnology. Globally, according to data from the ClinicalTrials.gov registry, the USA (2,196) and China (1,387) remain firmly in first and second place.
However, the world’s largest trial registry, ClinicalTrials.gov, does not capture all studies registered in China. The official Chinese registry reported around 4,900 studies for 2024.
The breakdown of interventional clinical trials involving Germany by disease area for studies that began in 2024 has not yet been released. Hoemke expects cancer (175 studies) and immune disease studies (109) to lead, followed by diabetes and metabolic disorders (39), and neurological diseases (31).
Germany aims to reclaim its former top position (2016: rank 2 with 641 studies) and is currently speeding up study initiation through standardized contract clauses and reduced bureaucracy—steps Spain implemented much earlier. From December 18, 2024, new contracts between clinics/practices and pharmaceutical companies for clinical studies must include the standard contract clauses established in September. The UK government is pursuing similarly ambitious goals. According to the vfa, most industry-sponsored studies at the roughly 30 major study sites took place in Berlin (231), Munich (140), and Hamburg (139).
The vfa notes that multiple factors influence where R&D facilities are expanded or newly established. These include good infrastructure, excellent cooperation partners such as universities and research institutions, highly qualified personnel, and flexible labor laws. Efficient processing of applications by authorities is also crucial, whether for animal experiments, clinical trials, drug approvals, or new research and production facilities.
Slightly different figures were reported in October by the European pharmaceutical federation EFPIA in a study conducted by IQVIA on 2023 studies. Here, Japan ranks third, ahead of Spain, among the 69% of studies funded exclusively by pharmaceutical companies. While the number of phase II to III studies remains consistently high, China has seen a sharp increase in phase I studies, which EFPIA expects will significantly shift future shares.
According to the vfa, its statistics focus on the number of trials initiated in 2024 and include only Phase I–IV studies, ensuring that medical device trials are excluded.


 FRAXA Ewsearch Organisation
FRAXA Ewsearch Organisation