
BioNTech‘s breast cancer ADC outperforms Roche’s Kadcyla
Data on the primary endpoint of BioNTech's/Duality Biologics' pivotal Phase III study of its HER2 antibody-drug conjugate BNT323/DB-1303 are not yet public, However, BioNTech says that progression-free survival is better than with Roche's second-line breast cancer therapy Kadcyla.
BioNTech’s/Duality Biologics’ Phase III pivotal study of trastuzumab pamirtecan (BNT323/DB-1303) compared the safety and efficacy of the ADCs with Roche’s Kadcyla, which carry the same HER2 antibody but different payloads. However, it also competes with Enhertu, which also uses a topoisomerase I payload (camptothecin derivative) and is marketed in Japan by Daiichi Sankyo and in collaboration with AstraZeneca in the rest of the world.
According to interim results of a Phase III study of BioNTech/Duality Biologics, trastuzumab pamirtecan performed better than Roche’s T-DM1 ADC, which is equipped with a non-cleavable thio-linker and the first-generation payload Maytansinoid DM1, in terms of the surrogate marker progression-free survival (PFS). and achieved a median PFS of 9.6 months in registration studies. It is not yet known whether BioNTech/Dual Biologics ADC was below or above the median PFS of 28.8 (DESTINY-Breast03 study) and 13.2 months (Low HER2, DESTINY-Breast06 study) achieved by Daiichi’s ADC Enhertu, as no data has been published by BioNTech to date. Trastuzumab pamirtecan shows strong bystander activity due to good membrane permeability, also a cleavable linker and a topoisomerase I payload.
However, as Roche’s Kadcyla, which is approved for metastatic breast cancer and as an neoadjuvant treatment, has recently seen strong growth in China in the adjuvant setting, BioNTech’s results pose a threat to Kadcyla’s global breast cancer sales of nearly US$2 billion. This is becauseBIONTech’s licencor Duality Biologics now intends to apply for marketing approval for trastuzumab pamirtecan in China.
As part of the head-to-head study with Roche’s Kadcyla, Duality Biologics recruited 228 breast cancer patients who had previously received chemotherapy and Roche’s anti-HER2 antibody trastuzumab (Herceptin).


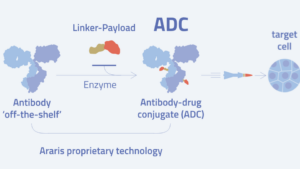 Araris Biotech AG
Araris Biotech AG Roche
Roche BioNTech SE
BioNTech SE