First in vivo CAR gene therapy trial in Europe approved
The Paul Ehrlich Institute (PEI) has approved expansion of the global INVISE Phase 1 clinical trial to Europe. The study aims to evaluate first-in-class gene therapy for the treatment of B-cell malignancies.
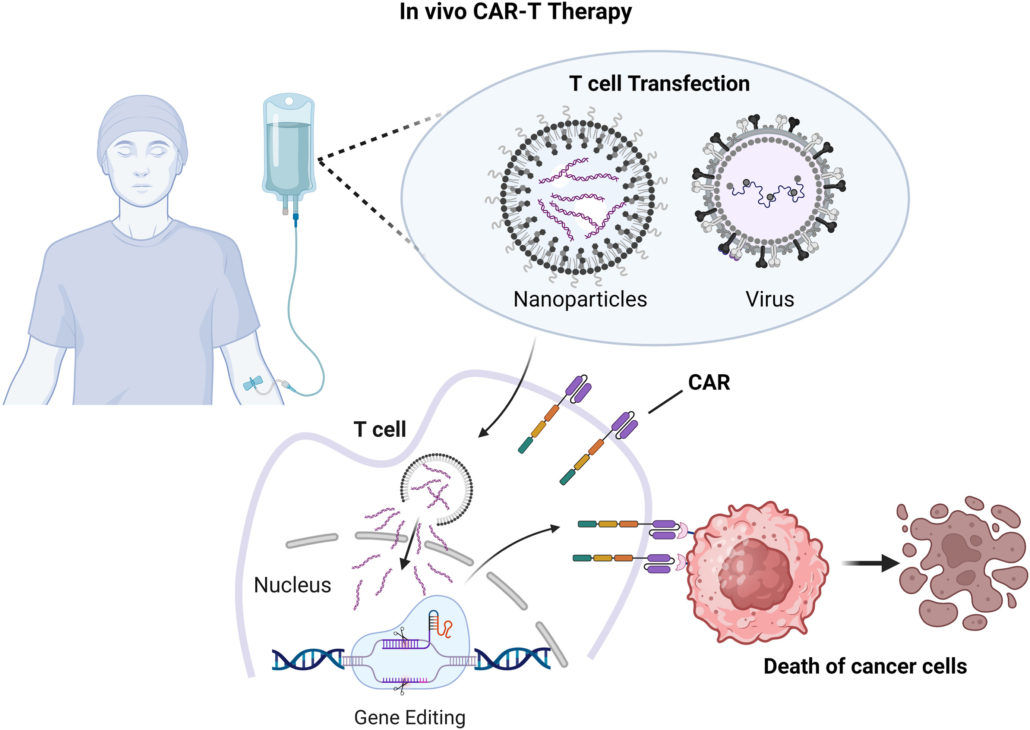
CAR-T therapies have already been approved in Europe, primarily using ex vivo approaches. However, with the recent approval by the German regulatory agency, the Paul Ehrlich Institute (PEI), the INVISE (INjectable Vectors for In Situ Engineering) study takes a step forward as the first clinical trial to evaluate in vivo CAR therapy in Europe. As announced by Interius, this global, multi-center trial marks the first human testing of INT2104 and is designed to assess the safety of intravenous administration in adults with refractory or relapsing B cell malignancies.
The first-in-class gene therapy INT2104 was engineered by Interius BioTherapeutics, Inc., a Philadelphia-based clinical-stage company developing targeted, programmable vectors for the precision delivery of genetic medicines. INT2104 specifically targets CD7-positive T and NK cells and delivers a CAR (chimeric antigen receptor) transgene to create effector CAR-T and CAR-NK cells in vivo. The CAR cells are then intended to target CD20-positive B cells for the treatment of B cell malignancies.
The approach aims to overcome the current limitations of ex vivo CAR T-cell therapy by creating therapeutic CAR cells directly in the patient’s body. Unlike ex vivo CAR-T therapies, INT2104 is a ready-to-use, single-dose treatment, given through intravenous infusion without the need for lymphodepletion or any special equipment or training. This will offer several advantages for patients, including faster treatment times, reduced hospital stays, lower costs, and less intensive preparation compared to traditional CAR-T therapies, making therapies more accessible and convenient.
“This approval highlights the strength of our preclinical data and the breakthrough potential of INT2104 to broaden access to CAR therapies”, said Interius President and CEO, Phil Johnson. “We remain committed to transforming the current treatment paradigm through clinical evaluation of our novel products, and the PEI’s approval marks a significant step.” The first patient was recently dosed in Australia, and more patients are expected to follow also in Europe soon.


 EC- press service
EC- press service AseBio
AseBio adobe stock photo - Toko
adobe stock photo - Toko