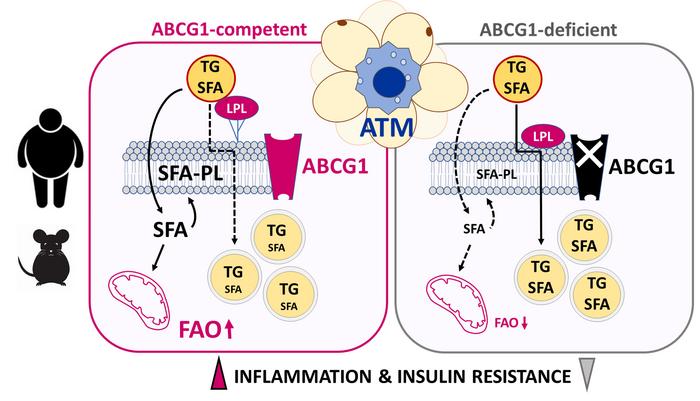
Membrane target triggers inflammation
Researchers at Sorbonne Paris have discovered that a membrane protein in adipocytes of obese mice and humans changes the lipid composition of adipose tissue macrophages rendering them more inflammatory and reducing insulin resistance.
Fatty acid rewiring impacts inflammation and insulin resistance in mouse obesity, French researchers from Sorbonne University have found out. The team headed by Wilfried Le Goff identified a lipid transport protein that seem to control how macrophages in adipose tissue respond to fatty acids that are abundant in high-energy diets.
Previous research suggested that lipid flux influences the activation of an inflammatory profile in adipose tissue macrophages (ATMs) triggering cardiometabolic diseases, including obesity, diabetes, NAFLD and ASCVD, along with the development of insulin resistance in obesity. Thus, learning more about how fatty acids are distributed in these macrophages could shed light on how obesity contributes to metabolic disorders. First author Veronique Dahik found that obese mice without the membrane Atp-binding cassette g1 (ABCG1) transporter protein in their macrophages had a lower inflammatory status and reduced insulin resistance, and that there is a similar relationship between inflammation and ABCG1 in human adipose tissue macrophages.
According to studies in mice, the loss of ABCG1 in the macrophages causes a rewiring of triglyceride fatty acid distribution, keeping proinflammatory lipids out of the macrophage membrane and rerouting them into lipid droplets where they are neutralised. “This contributed to a less metabolically activated ATM phenotype, which globally improved adipose tissue health and attenuated insulin resistance,” Dahik concluded. Abolition of this anti-inflammatory phenotype in Abcg1-deficient ATMs was achieved through restoration of lipoprotein lipase (Lpl) activity, thus delin- eating the importance of the Abcg1/Lpl axis in controlling ATM metabolic inflammation.
Le Goff has patented siRNAs blocking the ABCG1 gene and diagnostics predicting the shift to an obese metabolism.


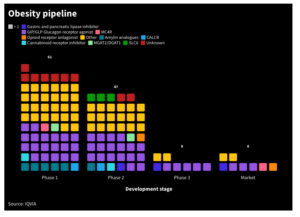 IQVIA
IQVIA
 Momoneymoproblemz - wikimedia
Momoneymoproblemz - wikimedia