Tubulis from Munich doses first Patient with own ADC linker technology
Tubulis doses first patient in phase 1/2a trial investigating ADC candidate TUB-040 in ovarian cancer and lung adenocarcinoma (NSCLC). TUB-040 is a NaPi2b-targeting Exatecan ADC based on Tubulis’ proprietary linker technology with better biophysical properties that demonstrated effective and durable responses in a range of preclinical models.
Tubulis, based in Martinsried near Munich, announced today that the first patient has been treated in its first Phase 1/2a trial (NAPISTAR 1-01, NCT06303505). The study is evaluating Tubulis’ next-generation antibody-drug conjugate (ADC) TUB-040 in patients with platinum-resistant high-grade ovarian cancer (PROC) or relapsed/refractory adenocarcinoma non-small cell lung cancer (NSCLC), who have exhausted other available treatment options. TUB-040 targets NaPi2b, a highly overexpressed antigen in ovarian cancer and lung adenocarcinoma. The candidate is the first to enter the clinic from the company’s growing pipeline and represents one of Tubulis’ two lead candidates developed using its proprietary suite of platform technologies, which enable the “creation of uniquely matched ADCs with superior biophysical properties” as the company claims.
The multicenter, first-in-human, dose escalation and optimization Phase 1/2a study aims to investigate the safety, tolerability, pharmacokinetics, and efficacy of TUB-040 as a monotherapy. The trial will be conducted in the US as well as the UK, Spain, Belgium, and Germany. Now, the first patient has been dosed in the US following IND approval by the FDA.
“Initiating our first clinical trial represents an important milestone for the entire Tubulis team and underscores our vision to innovate on all fronts of the ADC design for patient benefit”, said Dominik Schumacher, PhD, Chief Executive Officer and Co-founder of Tubulis. TUB-040 consists of a humanized, target-specific, Fc-silenced IgG1 antibody equipped with Tubulis’ proprietary Tubutecan linker-payload technology, which is based on P5 conjugation chemistry and the topoisomerase-1 inhibitor Exatecan. In a range of preclinical models, Tubulis was also able to show high and long-lasting anti-tumor responses, even at lower expression levels of NaPi2b, with an excellent safety and tolerability profile.
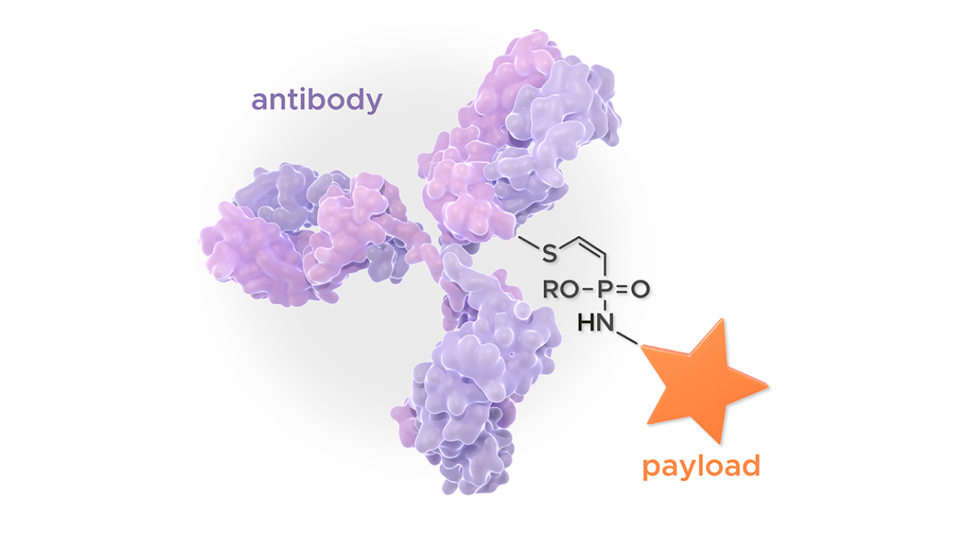
TUB-040 provides a special chemical technology for linkage: through a cleavable linker system based on the company’s proprietary P5 conjugation technology with a homogeneous Drug-Antibody-Ratio (DAR) of 8. P5 conjugation is a novel chemistry for cysteine-selective conjugation that enables ADC generation with unprecedented linker stability and biophysical properties. It originated from the fundamental work of Prof. Christian Hackenberger at the Leibniz-Forschungsinstitut für Molekulare Pharmakologie im Forschungsverbund Berlin e.V. (FMP), which unlocked the use of phosphorus chemistry for superior bioconjugation.
Tubulis attracted great financial support over the last few months from VC investors like Andera Partners, Nextech Invest, Biomed Partners and EQT Life Sciences among others and added another €128m series B2 round in early spring to a financing round from 2022 in the €60 millions beeing record high for Germany at this time.



 BioNTech SE
BioNTech SE Roche
Roche