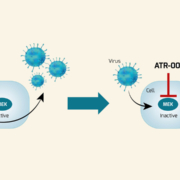
Viral pandemics are not a once-in-a-lifetime event but a constant threat looming over our heads. While the fight against SARS-CoV-2 is currently a top priority, we should not lose sight of what might come next. Broad-spectrum antiviral drugs like Atriva’s ATR-002, currently in Phase II clinical development for treating COVID-19, could be an effective measure in the fight against the current pandemic and future viral outbreaks.
ADVERTISEMENT
Roche has reported the failure of its IL-6R antagonist tocilizumab in patients with COVID-19 putting the efficacy of IL-6 inhibition at question.
Italian start-up Enthera Pharmaceuticals has raised €28m to advance restorative therapy for type 1 diabetes and inflammatory bowel disease.
Oncolytic viruses, viral vectors for gene therapy and viral-vectored prophylactic vaccines, are new, innovative, and already successful approaches for the treatment of severe diseases. The production of these viruses requires extensive experience and specially-designed GMP clean rooms. Vibalogics offers both.
SPCs offer up to five and a half years of additional protection after patent expiration for pharmaceuticals in order to compensate the patent holder’s inability to exploit their inventions while products undergo lengthy regulatory approvals.
Swiss Polyphor AG has strengthened its financial flexibility with an equity-linked financing.
Interview with Ian Harrow from the Pistoia Alliance, about how to improve life sciences data management with help of a new digital toolkit that makes data Findable, Accessible, Interoperable, and Reusable – in brief: FAIR.
Prior to Danish Genmab A/S’s recent licence option deal with AbbVie Inc, Rentschler Biopharma has extended its collaboration with Genmab. European Biotechnology spoke with Rentschler Biopharma SE’s SVP Global BD Federico Pollano about the CDMO’s expertise in DuoBody® molecules production and the expansion of the CDMO’s U.S. production site in Milford.
According to current information, the European Research Framework Programme "Horizon Europe" is likely to be among the indirect victims of the Corona pandemic: A large part of the funds earmarked will now go to the € 750 billion rescue fund.
As the process determines the product, product characteristics of biological drugs need to be analysed thoroughly right from the beginning of the drug development process. Early understanding of structure-function relationships, aggregation, microheterogenicity, and glycosylation, and more, helps minimise development risks.