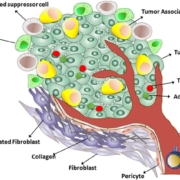
mRNA cancer neoantigen specialist BioNTech AG is set to buy all shares of Neon Therapeutics, Inc. expanding its pipeline of neoantigen-targeting therapies and getting foothold in the US.
ADVERTISEMENT
British researchers have identified several small molecule drugs that sensitized B cell cancers to chemotherapy and extended survival in mouse models.
Adaptimmune Therapeutics plc and Japanese Astellas Pharma, Inc. have signed a discovery partnership to develop off-the-shelf allogeneic T cell-based cancer therapies from stem cells.
Twelve years after Roche’s $6.8bn hostile takeover bid for Illumina, the companies have entered into a 15-year partnership to boost personalised NGS-based cancer testing.
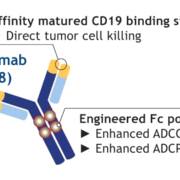
Incyte Corp. has signed a global license agreement for commercialisation of Morphosys AG’s anti-CD-19 programme tafasitamab.
In silico clinical trials pioneer Novadiscovery SA has raised €5m in a series A financing led by Swiss Debiopharm Innovation Fund.
Dreams of simple, reliable and scalable processes for the much-hyped cell therapy market have generated demand for cost-effective, GMP-compliant manufacturing methods. The drug-screening market is also hungry for material that will allow the establishment of human cell disease models. A growing number of companies are now trying to coax induced pluripotent stem cells (iPSCs) into specific cells needed for clinical trials. Others are seeking the same goal through direct transdifferentiation protocols. What method will prove superior?
Following a €1.5m seed financing in 2017, cancer specialist Alderaan Biotechnology SAS has raised €18.5m in a Series A round led by Advent France Biotechnology and Medicxi.
NorthSea Therapeutics BV has raised US$40m to provide proof-of-concept of its NASH drug icosabutate.
4SC AG and Merck KgaA have signed a supply agreement to start clinical tests of Merck’s checkpoint blocker avelumab plus 4SC’s HDAC I blocker domatinostat.