A newly forming industry association warns the European Commission not to block patients’ access to the emerging EU market for medicinal cannabis. At the BIO-Europe in Hamburg, European Biotechnology spoke with one of the initiators, Peter Homberg.
ADVERTISEMENT
The new company, named Augustine Therapeutics, is a Vlaams Instituut voor Biotechnologie (VIB) and Katholieke Universiteit (KU) Leuven spin-off. Funded with €4.2m from mainly local investors, Augustine’s first priority is finding new therapeutics for patients suffering from Charcot-Marie-Tooth disease, a hereditary motor and sensory neuropathy of the peripheral nervous system.
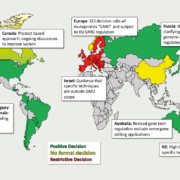
After years of regulatory deadlock concerning political decision making on genome-edited and genetically targeted mutational breeding, the European Council has demanded that the European Commission regulate new plant breeding techniques (NBT), such as oligonucleotid-directed mutagenesis (ODM), gene scissors (CRISPR), and others.
French Transgene SA said it has terminated its lead programme TG4010 in combination with chemotherapy and checkpoint inhibition.
The European biotech sector is flourishing – rising stock prices, big partnering deals, and some major M&A. Can someone please tell European fund managers that investing in biotech is simply mind over matter?!
Medical cannabis has become one of the most important economic topics in recent years! But, what makes this topic so relevant, and why is it so essential to found a European association? The legalisation of recreational and medicinal cannabis in different states has led to impressive economic progress in recent years. Through worldwide patient campaigns and global marketing strategies, the topic became a permanent information focus for social networks and politics at the end of 2018.
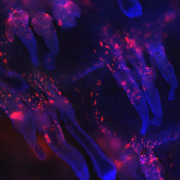
New research demonstrates that disruption of EGF receptor signalling allows commensal bacteria to invade hairs and cause skin lesions.
The European Circular Bioeconomy Fund, which aims to provide financing for innovative circular bioeconomy companies and projects in the EU and in countries associated with the Horizon 2020 programme, will be headed by Michael Brandkamp.
Madrid-based RNA specialist Bioncotech Therapeutics has entered into a Phase II clinical trial collaboration with a MSD subsidiary.
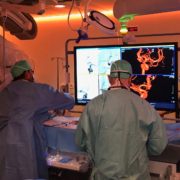
Swiss surgeons have significantly improved mechanical removal of blood clots in stroke patients by intra-arterial administration of the thromolytic urokinase.