Life sciences investment group LSP has launched the largest fund in Europe dedicated to late-stage medical technology assets, including diagnostics and digital health.
ADVERTISEMENT
Belgian start-up company Aelin Therapeutics NV secured the funds to push development of candidate drugs arising from its Pept-in protein knockdown platform, which harnesses protein aggregation to induce functional knockdown of target proteins.
Dutch llama antibody specialist ArgenX met the safety endpoint in a Phase II trial with ARGX 113 (efgartigimod), a first-in-class antibody that lowers pathogenic levels of IgG, in patients with the autoimmune disease myasthenia gravis.
In vitro diagnostics leader Ortho Clinical Diagnostics (Raritan, US) expands his assay portfolio with Sphingotec’s first-in-class biomarker test that predicts residual edema and re-hospitalization in patients with congestive heart failure.
The renewable energy sector is ramping up in a major way, but a host of infrastructure issues still need to be solved. One is that supply at times now outstrips demand, but there’s no way to store excess power. Turning it into an easy-to-store, energy-rich source like methane could solve the problem. A pivotal aspect of power-to-gas’ technology harnesses some tiny helpers usually found in some of the most extreme regions on the planet.
French based Advicenne has raised a €27m IPO on Euronext Paris, to develop paediatric-friendly therapeutics for the treatment of orphan renal and neurological diseases.
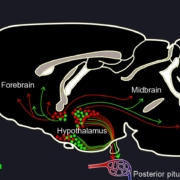
A European-Australian team of researchers has created a synthetic version of oxytocin that does not show the off-target effects mediated by its chemical analogon vasopressin.
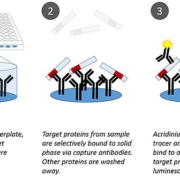
Rapid detection of total bacteria within 2½ h.
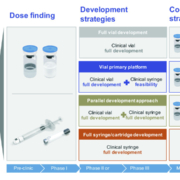
Considering the high development costs of medications and the time invested before a drug goes from discovery to market, it is critical to develop not only a solid drug development strategy, but a contemporary manufacturing strategy as well. A parallel development strategy could be one possible approach.
Roche company Foundation Medicine Inc. (FMI) has been granted FDA approval for a diagnostic multiplex tumour profiling assay, which identifies a broad range of tumour markers in one single measurement.