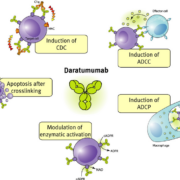
Genmab A/S has announced that its CD38-targeting Phase II candidate daratumab did not reach the expected ORR in non-Hodgkin’s lymphoma patients. Co-developer Janssen has decided to terminate development in three NHL subtypes.
ADVERTISEMENT
The evaluation of the European bioeconomy strategy will take longer than initially planned. According to information given at the Biostep Forum in Brussels, results won’t be available before next year and it is still unclear if there will be an update of the strategy adopted in 2012. Several upcoming expert and stakeholder meetings in 2017 will pave the way for a decision in early 2018.
The decision of the European Patent Office to examine all patent applications within 12 months can unintentionally block companies from filing for life sciences patents in Europe, says Gavin Recchia, Principal of one of Australia’s largest patent attorneys’ firms.
Polish drug developer Selvita has out-licenced its first lead compound to Berlin-Chemie Menarini, a company of the Menarini Group. Ongoing clinical trails with SEL 24 will be conducted the company listed on Warsaw Stock Exchange and taken over later this year by Menarini.
The EU’s opt-out clause for GM cultivation has missed its goal to accelerate EU market approval of safety-assessed genetically modified crops whilst giving member states the option to opt out from cultivation for political reasons.
Three of six new meds to be approved in Europe are orphan drugs and have been backed by the European Medicines Agency (EMA) this month.
Good news for EMD Serono, the US arm of German Merck: It’s PD-L1 blocker avelumab is the first drug that received FDA approval to treat the rare skin cancer Merkel Cell Carcinoma (MCC).
Scientists at EPFL in Lausanne have developed a semi-automated technology that may be a game-changer by making the characterisation of the 2,000 DNA-binding proteins much faster, more accurate, and efficient.
With around 2,500 attendees, Barcelona hosted the most successful BIO Europe Spring conference in the history of the EBD’s partnering event. Southern Europe demonstrated its growth ambitions in the life sciences.







 © Merck KGaA
© Merck KGaA
