Santhera licenses CF drug from Polyphor
Santhera Pharmaceuticals has licenced the inhaled neutrophil eleastase blocker POL6014 from Polyphor Ltd for CHF6.5m upfront and CHF121m in potential milestones.
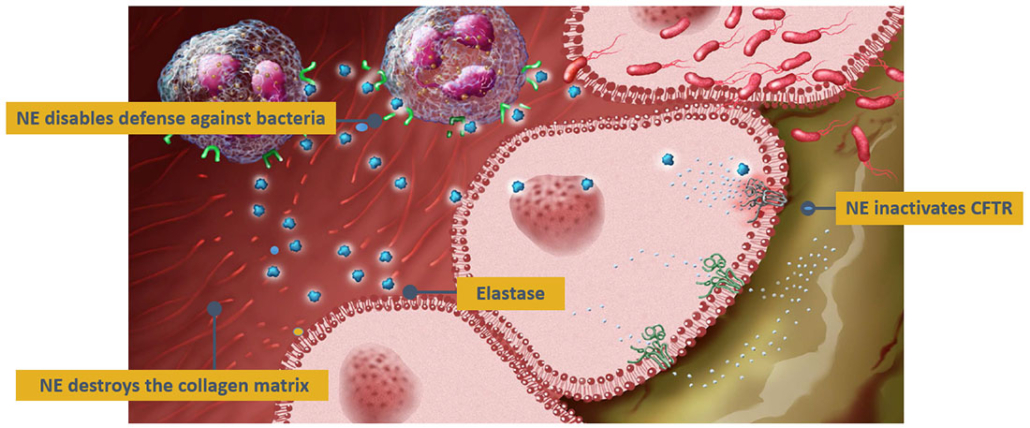
According to the exclusive global license agreement, Santhera has the right to develop and commercialise Polyphor‘s drug candidate in inflammatory lung diseases characterised by activity of the neutrophil protease such as non-cystic fibrosis bronchiectasi, alpha-1 antitrypsin deficiency, and primary ciliary dyskinesia as well as cystic fibrosis. Pol6041 is ready to enter Phase II trials in cystic fibrosis after having already passed first-in-man and single dose escalation Phase I trails without reported SAEs. The small molecule drug also triggered elastase reduction in sputum. Human neutrophil elastase degrades elastin and collagen, leading to lung tissue damage, increased mucus hyper-secretion and upregulates pro-inflammatory cytokines (IL-8) maintaining chronic bronchitis and persistent neutrophilia.
Santhera said that the licence agreement will expand its product pipeline in pulmonary diseases, where the company tries to receive US market access for its benzoquinone analogon idebenone as first-in-class treatment to slow the loss of respiratory function in patients with Duchenne muscular dystrophy. In Europe, the EMA rejected an MAA in this indication demanding more data.
Santhera will pay the upfront fee of CHF 6.5m in shares. Unter the agreement, potential future milestones of up to CHF 121m are payable in cash. In addition, Polyphor is entitled to tiered royalty payments from Santhera’s future net sales of POL6014 and to undisclosed milestone payments and royalties provided that Santhera advances the development and market entry of POL6014 in other pulmonary diseases.
Thomas Meier, CEO of Santhera, gave an outlook on the next clinical steps: "In a first step, we intend to execute a multiple ascending dose (MAD) tolerability trial during the second half of 2018 which has already been planned by Polyphor. In parallel, we will initiate discussions with EU and US regulators around the development program for POL6014 in CF and other indications." Phase I development had been co-financed through a US$3m fund of the Cystic Fibrosis Foundation.
So far, there are no cystic fibrosis drugs on the US$3.5bn market that can slow the loss of respiratory function in patients with cystic fibrosis.




