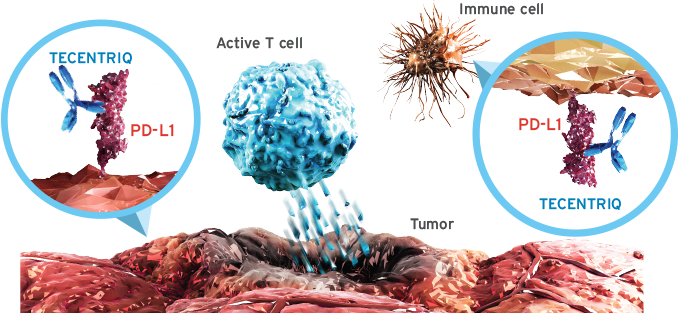
Roche presents biomarker test to identify NSCLC responders to Tecentriq
Researchers at Roche's US arms Genentech and Foundation Medicine Inc. (FMI) presented a liquid biopsy test that can be used to identify NSCLC patients who respond to Roche's PD-L1 blocker Tecentriq earlier than to FMI's tissue based tumour mutation burden (TMB) assay.
In Nature Medicine, the researchers reported that a mutation frequency of 16 mutations per megabase DNA or above that threshold predicted best if NSCLC patients responded to Roche’s checkpoint-inhibitor as measured by improvement of progression-free survival (PFS). Retrospective analysis of plasma samples from 211 patients were the basis for definfing the cut-off value that subsequently was validated on 583 tissue samples obtained from a Phase III trail. Genentech researchers hope that the blood-plasma based test will allow to identify responders earlier that with a tissue-based TMB assay developed by Foundation Medicine.
In the current setting, however, this would make few difference as Tecentriq is FDA-approved for treatment of patients with advanced urothelial carcinoma and patients with metastatic non-small cell lung cancer (NSCLC). Stage IV NSCLC patients have a poor prognosis, only 1-10% survive for 5 years, most die in any case. In these patients, Tecentriq, however may delay the time to death. As Tecentriq improved PFS and overall survival in patients with both high PD-L1 expression and bTMB of 16 or more, Roche is clinically validating the predictive power of the biomarker combination in Phase II/III tests which aim at establishing Tecentriq as first-line treatment in the lucrative but highly competitive NSCLC market.




