Genfit set to raise $100m in Nasdaq IPO
French Genfit SA has registered at SEC for an Nasdaq IPO of ADS in conjunction with private placements in Europe and the US.
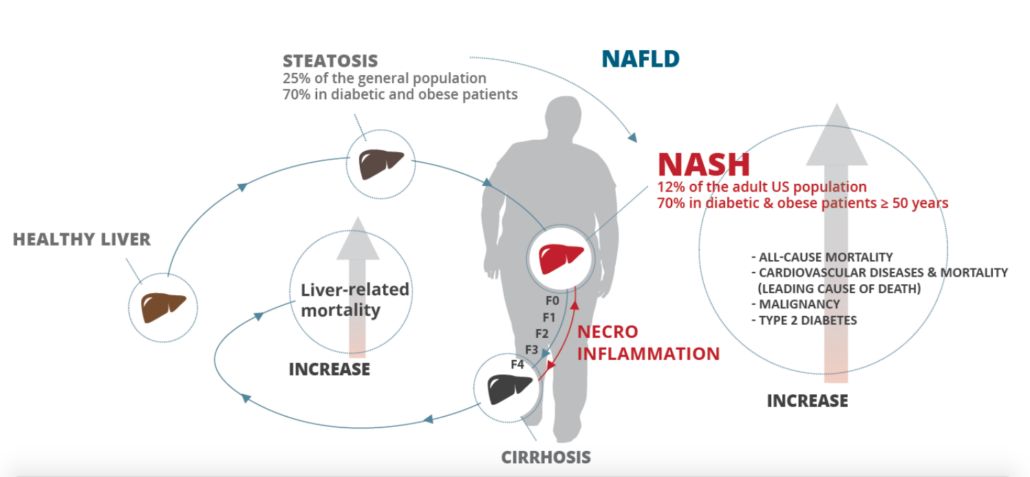
Proceeds of the dual listing will be used to finance the roll-out of Genfit’s lead molecule elafibranor, currently in Phase III testing to treat NASH (non-alcoholic steatohepatitis), a condition many drug developers added to its pipelines in the past five years. Results from the pivotal trial with the dual PPAR? and PPAR? agonist are expected to be announced at the end of 2019/start of 2020. Genfit will also finance another Phase III study with the candidate primary biliary cholangitis expected to start later this year.
Elabibranor has been shown to resolve steatosis without worsening fibrosis. It has a complemantary mode of action that leads to higher insulin sensitivity, and less steatosis, inflammation/oxidative stress, hepatocyte damage, and fibrosis as well as a lower NAS score.
Since 2006, Genfit is listed on Euronext. SVB Leerink and Barclays are acting as joint global coordinators for the Global Offering and joint bookrunners for the US Offering. Roth Capital Partners and H.C. Wainwright & Co. are acting as co-managers for the US Offering. Bryan, Garnier & Co. Limited and Natixis are acting as joint bookrunners for the European Private Placement.



 Unsplash+
Unsplash+